Abstract
How embryos are cultured, in groups or individually, can influence their development and have other unforeseen impacts on subsequent assisted reproductive technologies. Although a group culture of embryos improves the blastocyst formation rates, this can create conditions wherein separate blastocysts may fuse. This fusion of 2 blastocysts can create unique logistic issues for embryo biopsy and genetic analysis. New culture approaches have emerged to facilitate individual embryo culture without losing the benefit of the group culture approach. Unique culture dishes and adjustments of laboratory culture/embryo handling protocols offer possible solutions to minimize or avoid blastocyst fusion.
Key Words: blastocyst, conjoined, fused, PGT, embryo biopsy
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/posts/xfre-d-20-00212
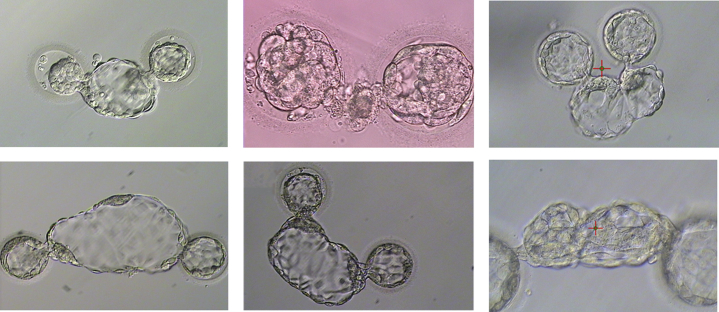
Existing literature indicates that a group culture of embryos in a variety of species, including humans, yields superior embryo development compared with individual culture. (1, 2) However, group embryo culture can create unique issues when attempting to use various selection approaches to identify or rank embryos for subsequent transfer such as preimplantation genetic testing (PGT). When culturing groups of embryos together to the blastocyst stage for biopsy, the presence of hatching blastocysts can result in the joining of 2 separate embryos (Fig. 1). This has been reported to occur at a rate of approximately 1:1500 embryos (3); although, the rate may more or less depend on the culture conditions. For example, the breaching of embryos on days 1–4 (before the blastocyst stage), emergence of novel embryo-specific culture dishes that promote the proximity of embryos to each other, or increased use of uninterrupted culture approaches wherein embryos are not physically separated before or during blastocyst hatching may exacerbate this phenomenon. In a network of related 11 in vitro fertilization laboratories utilizing group culture of up to 5 embryos per approximately 30 μl in an embryo-specific dish (Mini GPS, Cooper Surgical, Målov, Denmark) that uses rounded bottom microdrops to promote cell proximity, along with breaching on day 3 and the use of sequential culture media with embryos rinsed/moved on days 3 and 5; the incidence of blastocyst fusion over a 2-year period was observed 10 times during the culture of over 80,200embryos and over 40,300 blastocysts biopsied (over 11,500 oocyte retrievals) (Swain JE, unpublished data).
Fig 1.
Examples of fused blastocysts during group culture that were breached on day 3 during preparation for blastocyst biopsy.
With 2 conjoined blastocysts, the issues regarding biopsy and subsequent chromosomal analysis are readily apparent; how can a representative sample of a blastocyst and accurate corresponding results be guaranteed? Another question that emerges is whether these blastocysts should be utilized at all for embryo transfer and how best to counsel patients.
It is fairly straightforward for a skilled embryologist to separate 2 blastocysts with fused trophectoderm cells because this is similar to the normal biopsy process. Care is taken to try to separate the blastocysts along the point of fusion, if this can be visualized. This is achieved normally by the use of a laser, though mechanical separation using microtools is also feasible. However, some conjoined blastocysts are so severe that it is unclear which cells belong to which embryo (Fig. 1). During the separation of fused blastocysts, care is taken to avoid any damage to the inner cell mass (ICM) if it is extruded from the zona pellucida and near the point of fusion. If the ICM is involved in the fusion or is too close to the point of fusion, separation becomes more difficult and may not be feasible and subsequent use is not advised. The embryologist must often make this decision based on what is observed and deemed safe.
When conjoined blastocysts have been separated, each with their own intact ICM, various options exist. One approach is that if PGT was planned, no biopsy is performed. In such cases, the 2 resulting blastocysts can be frozen and kept for future use and prioritized lower than any resulting biopsied/normal/unaffected blastocysts that may have resulted from that cycle. The patient can be informed of this strategy. In this scenario, no biopsy of the hatched portion of the blastocysts is advised because of the inability to ensure that any biopsied trophectoderm cells are representative of a particular embryo. Of note, this approach may not be a suitable option for cases of PGT-monogenic or PGT-structural rearrangements.
When fused blastocysts are separated, it is often possible to perform a subsequent biopsy at a point away from the prior blastocyst fusion point. Although this approach would presumably give an accurate representation of the majority of the blastocyst, the issue remains of whether this is representative truly of the rest of the embryo. However, the prior blastocyst fusion event is unique in that it creates a chimeric embryo, albeit some more severe than others (amount of cell crossover, ICM vs. trophectoderm and creates a unique potential clinical outcome. Even with biopsy samples taken from an area away from the fusion point,3 depending on the diagnosis of the 2 blastocysts, outcomes can be compromised. If both the embryos are abnormal, there may be no issue because both the embryos would likely be unavailable for transfer. If both the embryos are normal or if 1 blastocyst is normal and 1 is abnormal, this creates a conundrum. A selection strategy deprioritizing the separated blastocysts and thorough counseling of the patient for the possible risks of misdiagnosis or other adverse outcomes is recommended.
A final approach would be that the conjoined blastocyst would be left intact and simply discarded because of the mixing of cells and uncertainty of the impact of the resulting offspring. This could potentially result in viable embryos being discarded or the patient being left with nothing to transfer. This is an inherent risk also present with embryo biopsy and PGT. While some may argue that leaving the blastocysts joined and having them available for transfer is a feasible option, this arguably creates a riskier outcome because of the risk of monochorionic, dizygotic pregnancy, and chimerism. While reports exist of transfers using a single blastocyst with 2 apparent ICMs (4), transferring 2 conjoined blastocysts presents a considerably different scenario.
Interestingly, with emerging approaches aimed at non-invasively assessing the embryo quality, such as those examining the chromosomal complement of blastocysts through sampling of blastocoel fluid or culture media (5, 6, 7, 8, 9), embryo-specific culture dishes may also be a part of the solution to avoid the fusion of blastocysts. Novel culture platforms aimed at optimizing the microenvironment to promote embryo development that permit individual embryo culture or even group embryo culture without direct embryo contact exist (10, 11). Until these approaches are more mainstream, methods to minimize blastocyst fusion include breaching at the blastocyst stage, minimizing the number of embryos cultured in groups, breaching at the opposite sides of the embryos if performed before blastocyst formation to reduce the chance for the 2 trophectoderm masses coming into contact, moving or separating embryos during culture (on day 5) to avoid fusion events when hatching begins, or using individual culture or dishes that separate the embryos for the entire culture period.
Footnotes
Disclosures: J.E.S. has nothing to disclose.
References
- 1.Reed M., Woodward B., Swain J.E. Single or group culture of mammalian embryos: the verdict of the literature. J Reprod Stem Cel Biol. 2011;2:77–87. [Google Scholar]
- 2.Ebner T., Shebl O., Moser M., Mayer R.B., Arzt W., Tews G. Group culture of human zygotes is superior to individual culture in terms of blastulation, implantation and life birth. Reprod Biomed Online. 2010;21:762–768. doi: 10.1016/j.rbmo.2010.06.038. [DOI] [PubMed] [Google Scholar]
- 3.Schiewe M.C., Whitney J.B., Anderson R.E. Potential risk of monochorionic dizygotic twin blastocyst formation associated with early laser zona dissection of group cultured embryos. Fertil Steril. 2015;103:417–421. doi: 10.1016/j.fertnstert.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Meintjes M., Guerami A., Rodriguez J.A., Crider-Pirkle S., Madden J.D. Prospective identification of an in vitro-assisted monozygotic pregnancy based on a double-inner-cell-mass blastocyst. Fertil Steril. 2001;76(Suppl 1):172S–173S. [Google Scholar]
- 5.Liu W., Liu J., Due H., Ling J., Sun X., Chen D. Non-invasive pre-implantation aneuploidy screening and diagnosis of beta thalassemia IVSII654 mutation using spent embryo culture medium. Ann Med. 2017;49:319–328. doi: 10.1080/07853890.2016.1254816. [DOI] [PubMed] [Google Scholar]
- 6.Shamonki M.I., Jin H., Haimowitz Z., Liu L. Proof of concept: preimplantation genetic screening without embryo biopsy through analysis of cell-free DNA in spent embryo culture media. Fertil Steril. 2016;106:1312–1318. doi: 10.1016/j.fertnstert.2016.07.1112. [DOI] [PubMed] [Google Scholar]
- 7.Gianaroli L., Magli M.C., Pomante A., Crivello A.M., Cafueri G., Valerio M. Blastocentesis: a source of DNA for preimplantation genetic testing. Results from a pilot study. Fertil Steril. 2014;102:1692–1699.e6. doi: 10.1016/j.fertnstert.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 8.Rubio C., Navarro-Sánchez L., García-Pascual C.M., Ocali O., Cimadomo D., Venier W. Multicenter prospective study of concordance between embryonic cell-free DNA and trophectoderm biopsies from 1301 human blastocysts. Am J Obstet Gynecol. 2020;223:751.e1–751.e13. doi: 10.1016/j.ajog.2020.04.035. [DOI] [PubMed] [Google Scholar]
- 9.Huang L., Bogale B., Tang Y., Lu S., Xie X., Racowsky C. Noninvasive preimplantation genetic testing for aneuploidy in spent medium may be more reliable than trophectoderm biopsy. Proc Natl Acad Sci USA. 2019;116:14105–14112. doi: 10.1073/pnas.1907472116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith G.D., Takayama D., Swain J.E. Rethinking in vitro embryo culture: new developments in culture platforms and potential to improve assisted reproductive technologies. Biol Reprod. 2012;86:62:1-10. doi: 10.1095/biolreprod.111.095778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swain J.E., Smith G.D. Advances in embryo culture platforms: novel approaches to improve preimplantation embryo development through modifications of the microenvironment. Hum Reprod Update. 2011;17:541–557. doi: 10.1093/humupd/dmr006. [DOI] [PubMed] [Google Scholar]