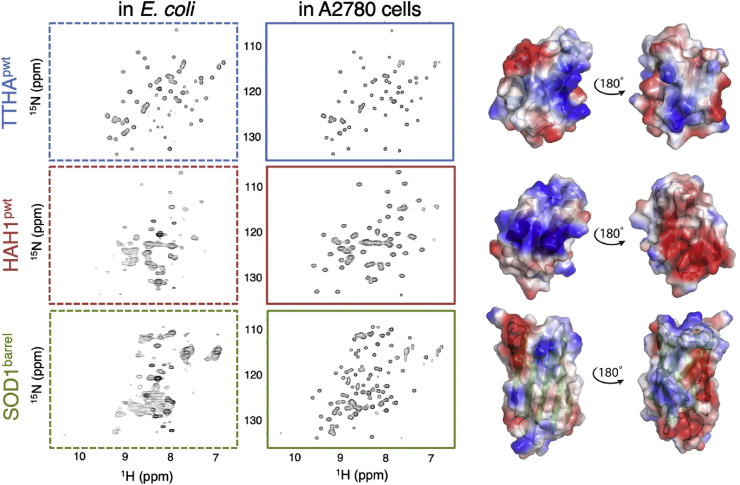
Fig. 2.
Improved in-cell NMR spectra in A2780 cells compared to E. coli. HMQC spectra of TTHApwt (blue frames), HAH1pwt (red frames) and SOD1barrel (green frames) in E. coli (dashed frames) and in live A2780 cells (solid frames). In E. coli, both HAH1pwt and SOD1barrel signals are severely broadened due to a high amount of interactions with the complex E. coli cytoplasm, i.e. the signals are barely visible in the HAH1pwt and SOD1barrel spectra even though the protein concentration is similar in all E. coli samples, as detected from the lysate signal intensity. In mammalian cells, all three proteins show significant improvement of the in-cell spectral properties, although still with varying line width. N.B. the contour levels differ in the E. coli dataset in order to visualise the broad low-intensity peaks of HAH1pwt and SOD1barrel. Right: the precited structures of TTHApwt (mutations templated on PDB id: 2ROE), HAH1pwt (mutations templated on PDB id: 1TL5) and the crystal structure of SOD1barrel (PDB id: 4BCZ) are shown. The calculated surface charge distribution is projected on the structure surfaces, with blue corresponding to positive charge density and red to negative, highlighting the differences in both charge distribution and charge clustering. Especially between the structural homologues TTHApwt and HAH1pwt severely broadened peaks. Even so, the line widths of the mammalian proteins narrow up in the cell lysates, showing that the Drot in the human cytosol is still reduced, albeit not as much as in E. coli (SI, Fig. S1). The results show thus that the mammalian cytosol affects the internalised proteins less than the E. coli cytosol, even to the extent that the protein-specific spectral characteristics are hard to distinguish at first sight.