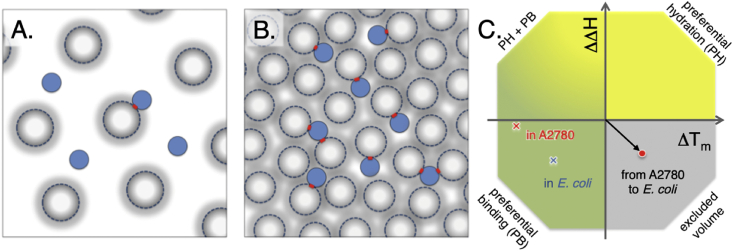
Fig. 7.
Snapshot representation of human and bacterial cytosol and thermodynamic classification of the effects from complex crowding solvents. A. A cartoon of the human cytosol with 60 mg/ml macromolecular concentration, here represented by ‘spherical’ proteins with the distribution of radii represented as soft edges. From relaxation data we can estimate that approximately 25% of the bacterial protein TTHApwt are involved in transient complexes at any given time point. B. The 6 times higher charge dependence on ηapp indicated 6 times higher macromolecular concentration in E. coli, and this results in that TTHApwt at any given time point is involved in a transient complex, and in more than 10% of the time it is involved in the formation of a transient trimer, with the slightly smaller proteins in the E. coli cytosol. C. The effects from altering the solvent can be classified from the effects on unfolding enthalpy (ΔH) and melting temperature (Tm), following the protocol by Ebbinghaus (Senske et al., 2014). The effects on SOD1barrel stability when comparing data from buffer to data from the A2780 cytosol is shown as a red ‘x’, and the corresponding effect when comparing to data from E. coli cytosol is marked as a blue ‘x’. Comparing human A2780 cell data to E. coli (red sphere) data results in a reduction in ΔH accompanied by an increase in Tm that can be classified as an excluded volume effect accompanied with increased transient binding, in line with an increased macromolecular concentration in the E. coli cytosol. The figure design is adapted from Senske et al. (Senske et al., 2014).