Abstract
Objective
Cerebral mitochondrial dysfunction is a key mediator of neurologic injury following cardiac arrest (CA) and is regulated by the balance of fusion and fission (mitochondrial dynamics). Under stress, fission can decrease mitochondrial mass and signal apoptosis, while fusion promotes oxidative phosphorylation efficiency. This study evaluates mitochondrial dynamics and content in brain tissue 24 h after CA between two cardiopulmonary resuscitation (CPR) strategies.
Interventions
Piglets (1 month), previously randomized to three groups: (1) Std-CPR (n = 5); (2) HD-CPR (n = 5; goal systolic blood pressure 90 mmHg, goal coronary perfusion pressure 20 mmHg); (3) Shams (n = 7). Std-CPR and HD-CPR groups underwent 7 min of asphyxia, 10 min of CPR, and standardized post-resuscitation care. Primary outcomes: (1) cerebral cortical mitochondrial protein expression for fusion (OPA1, OPA1 long to short chain ratio, MFN2) and fission (DRP1, FIS1), and (2) mitochondrial mass by citrate synthase activity. Secondary outcomes: (1) intra-arrest haemodynamics and (2) cerebral performance category (CPC) at 24 h.
Results
HD-CPR subjects had higher total OPA1 expression compared to Std-CPR (1.52; IQR 1.02–1.69 vs 0.67; IQR 0.54−0.88, p = 0.001) and higher OPA1 long to short chain ratio than both Std-CPR (0.63; IQR 0.46−0.92 vs 0.26; IQR 0.26−0.31, p = 0.016) and shams. Citrate synthase activity was lower in Std-CPR than sham (11.0; IQR 10.15–12.29 vs 13.4; IQR 12.28–15.66, p = 0.047), but preserved in HD-CPR. HD-CPR subjects had improved intra-arrest haemodynamics and CPC scores at 24 h compared to Std-CPR.
Conclusions
Following asphyxia-associated CA, HD-CPR exhibits increased pro-mitochondrial fusion protein expression, preservation of mitochondrial mass, improved haemodynamics and superior neurologic scoring compared to Std-CPR.
Institutional protocol number
IAC 16-001023.
Keywords: Cardiac arrest, Cardiopulmonary resuscitation, Mitochondria, Fusion, Fission, Dynamics, Neurologic outcomes
Introduction
Pediatric in-hospital cardiac arrests affect >15,000 children in the US each year, more than half do not survive to hospital discharge, and permanent brain injury is common among those who survive.1, 2, 3 A critical convergence point for neurologic injury following ischaemia-reperfusion injury is cerebral mitochondrial dysfunction, which mediates ongoing secondary injury and cell death.4, 5, 6 The impact of intrinsic cerebral mitochondrial quality control in response to mitochondrial dysfunction on neurologic outcomes after cardiac arrest is a novel and promising area of study.7, 8, 9, 10
Mitochondrial function is crucial to cellular health through a variety of cellular processes, including energy production, calcium signaling,11 reactive oxygen species generation12, 13 and apoptosis.14 Under basal conditions and in response to cell stressors and injury, mitochondrial quality control – ensuring appropriate numbers of normally functioning mitochondria and removing damaged ones – is an essential component of maintaining optimal mitochondrial and cellular function.15, 16 The regulation of mitochondrial morphology, also known as mitochondrial dynamics, is a key element of this process and is accomplished through the expression and activity of specific proteins and their post-translational modifications that dictate a constant interplay of fusion, fission, and mitophagy.15, 17 Mildly dysfunctional mitochondria can fuse with healthy mitochondria to optimize complementation, mediate oxidative stress, and improve oxidative phosphorylation; severely dysfunctional mitochondria will be sequestered and recycled through fission and mitophagy.16, 18, 19 Under stress, fusion may salvage bioenergetic output, redistribute mtDNA, and protect cellular components. However, with prolonged and severe stress, fission is activated, which causes mitochondrial fragmentation, increases reactive oxygen species (ROS) production, and triggers mitochondrial apoptotic pathways.19, 20
Our group has previously shown that in pediatric porcine models of asphyxia-associated cardiac arrest, a haemodynamic-directed CPR strategy (HD-CPR) improved mitochondrial respiration and survival with favorable neurologic outcome compared to a standard, guideline-based CPR strategy (Std-CPR).21, 22, 23 To understand the effect of cardiac arrest and cardiopulmonary resuscitation (CPR) on mitochondrial dynamics in the immature brain, we performed a protein analysis of cerebral mitochondrial dynamics and measured mitochondrial content in cerebral cortex 24 h after these two distinct CPR strategies. We additionally evaluated intra-arrest haemodynamics and cerebral performance category (CPC) among survivors of CA. We hypothesized that 24 h after return of spontaneous circulation (ROSC), cerebral cortical tissue from animals resuscitated with HD-CPR would have protein expression consistent with mitochondrial fusion and a preservation of mitochondrial content, as well as superior intra-arrest haemodynamics and improved post-arrest clinical neurologic scoring compared to Std-CPR.
Materials and methods
Cohort description
These experiments were performed as a component of a previously reported pre-clinical trial in which animals were randomized to HD-CPR vs Std-CPR.22 In that study, 7 of 10 animals treated with HD-CPR and 5 of 12 animals treated with Std-CPR survived to 24 h. The first five animals in each group that survived to 24 h were included in the present study, as were seven anesthetized shams, as described below.
Animal preparation
All animal procedures were approved by the Institutional Animal Care and Use Committee at the Children’s Hospital of Philadelphia (CHOP) and were conducted in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals. Healthy one-month-old, female swine, Sus scrofa domesticus, (8−11 kg) were anesthetized and mechanically ventilated using room air and titrated isoflurane (1.0–2.5% inspired concentration) with a tidal volume of 10–12 ml/kg and positive end-expiratory pressure of 6 cm H2O; respiratory rate was titrated to maintain end-tidal carbon dioxide (ETCO2) at 38–42 mmHg.22, 24, 25
Measurements
Femoral arterial and venous sheaths were placed and micromanometer-tipped pressure transducers were advanced to the aorta and right atrium for continuous measurement. Coronary perfusion pressure (CoPP) during CPR was calculated as the difference between the aortic diastolic pressure (DBP) and the right atrial pressure. The Philips Heart Start MRx defibrillator (Philips Medical Systems, Andover, MA) provided audiovisual feedback to the provider for rate, compression depth and chest wall recoil during CPR.22
Model justification
This study used an established porcine model because of similarities in neurodevelopmental and chest compression characteristic between swine and humans.26, 27 The experimental model was meant to simulate an asphyxial event preceding CA, as many pediatric in-hospital cardiac arrests have a respiratory etiology.1, 28, 29
Experimental protocol
Briefly, anesthetized animals underwent 7 min of asphyxia produced by endotracheal tube clamping followed by induction of ventricular fibrillation (VF) to ensure a standard 10-minute period of CPR that would only be terminated by defibrillation (Fig. 1). More detailed experimental protocols have been reported in previous publications.21, 22, 25 Compressions were provided with a target rate of 100 per minute and ventilations at 6 per minute with 100% oxygen. Animals were previously randomized to HD-CPR, standard, guideline-based resuscitation (Std-CPR), or anesthetized shams. In HD-CPR, chest compression force was adjusted to target a systolic blood pressure (SBP) of 90 mmHg and vasopressors were given to maintain CoPP greater than 20 mmHg. Beginning two minutes into CPR, vasopressors were administered if CoPP <20 mmHg as follows: adrenaline (0.02 mg/kg), adrenaline (0.02 mg/kg), and vasopressin (0.4 U/kg), with minimal dosing interval of 1 min following adrenaline doses, and 2 min following vasopressin administration. In Std-CPR, target depth was one third of the anterior-posterior chest diameter based on real-time feedback from the CPR quality-monitoring defibrillator. After 2 min of CPR, adrenaline dosing of 0.02 mg/kg was given and repeated every 4 min. In both treatment groups, beginning 10 min after the start of CPR, defibrillation was attempted every two minutes as necessary until ROSC or a maximum of 20 min of CPR. After ROSC, animals received standardized post-arrest intensive care. Shams were anesthetized and instrumented with central venous and arterial catheters, underwent protocolized, similar anesthetic exposure with inhaled isoflurane (≈1.0%). Blood pressure, end-tidal carbon dioxide, oxygen saturation, temperature, and anesthesia were maintained by using the identical explicit post-ROSC protocol as for the treatment groups. Following emergence from anesthesia, all animals were extubated and monitored for 24 h, where downstream outcome metrics were performed by members of scientific team blinded to treatment status.
Fig. 1.
Schematic of experimental protocol. Definitions of abbreviations: CPR cardiopulmonaryresuscitation; ETT = endotracheal tube; VF = ventricular fibrillation; Std = Standard, CPR = cardiopulmonary resuscitation; HD = haemodynamic-directed; SBP = systolic blood pressure; AP = anterior–posterior; CoPP = coronary perfusion pressure; ROSC = return of spontaneous circulation.
Sample acquisition
At 24 h post-arrest, animals were re-anesthetized and intubated. A bilateral craniectomy was performed to expose the brain, and cortical tissue was rapidly extracted and snap frozen in liquid nitrogen. Animals were humanely euthanized while under general anesthesia. All experimental procedures were performed on previously banked tissue of animals that survived to 24 h after CA.
Laboratory analysis
Frozen tissue whole cell lysate preparation
Frozen cerebral cortex tissue was homogenized (5% wt:vol) with 4 rounds of 5 strokes in a Potter-Elvehjem teflon-glass homogenizer in RIPA buffer (#89901, Pierce, Rockford, IL, USA) with protease inhibitor (AEBSF, Aprotinin, Bestatin hydrochloride, E-64, Leupeptin, Pepstatin A) (#P8340, Sigma Aldrich, St. Louis, MO, USA) and 5 mM NaF (#201154, Sigma Aldrich, St. Louis, MO, USA) as a phosphatase inhibitor. The homogenate was centrifuged at 12,000 × g for 10 min, the supernatant whole cell lysate was preserved and the pellet discarded. Total protein was quantified using the BCA Protein Assay Kit (#23228; Pierce, Rockford, IL, USA).
Western blots
Equal amounts of lysate protein were denatured in 4× Laemmli sample buffer with 1:10 dithiothreitol (DTT) (D0632-1G, Sigma Aldrich, St. Louis, MO, USA), separated by SDS–polyacrylamide gel electrophoresis (12% Bis-Tris, #NP0349BOX, ThermoFisher, Carlsbad, CA, USA), and transferred to nitrocellulose membranes (#88024, Thermo Scientific, Rockford, IL, USA). Membranes were blocked with Odyssey Blocking Buffer (#927-40000, Li-Cor, Lincoln, NE, USA) and incubated with primary antibody overnight for the following: DRP1 (1:15K, #32898, Santa Cruz Biotechnology, Santa Cruz, CA, USA), FIS1 (1:1 K, #10956-1-AP, Proteintech, Rosemont, IL, USA), OPA1 (1:5 K; #NB110-55290, Novus Biologicals, Littleton, CO, USA), MFN2 (1:1 K, #PA5-42171, Invitrogen, Rockford, IL, USA) and alpha-tubulin (1:20 K; #ab7291, Abcam, Cambridge, MA, USA). After washing, membranes were stained with IRDye secondary antibody (1:15 K, #925-32211, Li-Cor, Lincoln, NE, USA) for 1 h, then imaged using an Odyssey Scanner (Li-Cor). Relative band densities were determined by digital densitometry using Li-Cor Odyssey Application Software v3.
Citrate synthase activity
Preserved chamber contents from previously performed high-resolution respirometry were stored at −80 °C and analyzed for citrate synthase (CS) activity quantification (Citrate Synthase Assay Kit, CS0720; Sigma). CS activity (μmol/mL/min) was used as an index of mitochondrial content.5, 22, 30
Porcine cerebral performance category
Porcine Cerebral Performance Category was independently determined 24 h after ROSC by two blinded, trained investigators. The scoring system is as follows: 1 is normal (no difficulty standing, walking, etc.); 2 is mild disability (can stand, but unsteady, slow to respond, drinking, not eating); 3 is severe disability (awake but not responding, cannot stand, walk, eat, drink); 4 is comatose; and 5 is dead.31, 32
Statistical outcomes and analysis
The primary outcome of this study was mitochondrial dynamics protein expression and mitochondrial mass in cerebral cortex at 24 h after ROSC. Secondary outcomes include intra-arrest haemodynamics and cerebral performance category as a measure of neurologic function 24 h after ROSC. Outcomes were compared between subjects that received HD-CPR and Std-CPR after asphyxial cardiac arrest and sham animals that received equal anesthetic time.
Statistical analysis was performed using GraphPad Prism v7 (GraphPad, La Jolla, CA) and Stata Version 14 (StataCorp, College Station, TX). Protein expression, mitochondrial content and cerebral performance category were not assumed to have normal distributions and were statistically evaluated for differences using one-way nonparametric ANOVA (Kruskal-Wallis) followed by a Dunn’s multiple comparisons test. Results are expressed as medians with interquartile ranges followed by p values of Dunn’s multiple comparisons test.
Haemodynamic data were recorded at a high sampling rate during CPR using PowerLab (ADInstruments, Colorado Springs, CO) and the mean of each 15-second epoch was then calculated. The haemodynamic data from the first ten minutes of CPR (excluding the first minute to account for the typical period of chest wall molding) were compared between the two CPR groups with a generalized estimating equation regression model accounting for animal-level clustering of data points.
Results
Intra-arrest haemodynamic measurements
During CPR, mean arterial pressure was higher with HD-CPR than Std-CPR (point estimate +8.1 [CI95: 1.4, 14.8] mmHg; p = 0.018), as were diastolic blood pressure (+6.1 [CI95: 0.01, 12.1]; p = 0.049) and coronary perfusion pressure (+6.2 [CI95: 0.2, 12.2] mmHg; p = 0.042) (Fig. 2). There were no significant differences in systolic blood pressure or right atrial pressure between groups.
Fig. 2.
Increased intra-arrest haemodynamic measurements in HD-CPR versus Std-CPR in animals that survived to 24 h after CA.
(A) Invasive mean arterial pressure monitoring during CPR period. Time zero represents initiation of chest compressions. Error bars represent SEM. MAP was higher with HD-CPR compared to Std-CPR (point estimate +8.1 [CI95: 1.4, 14.8] mmHg; p = 0.018).
(B) Calculated coronary perfusion pressure as DBP minus RAP. Time zero represents initiation of chest compressions. Error bars represent SEM. CoPP was higher with HD-CPR compared to Std-CPR (point estimate +6.2 [CI95: 0.2, 12.2] mmHg; p = 0.042).
(C) Invasive systolic blood pressure monitoring during CPR period. Time zero represents initiation of chest compressions. Error bars represent SEM. No statistical difference was found between HD-CPR and Std-CPR. (point estimate +8.43 [CI95: −11.9, 28.8] mmHg; p = 0.416).
(D) Invasive diastolic blood pressure monitoring during CPR period. Time zero represents initiation of chest compressions. Error bars represent SEM. DBP was higher with HD-CPR compared to Std-CPR (point estimate +6.1 [CI95: 0.01, 12.1] mmHg; p = 0.049).
Expression of fusion and fission proteins
Animals treated with HD-CPR had a near three-fold median increase in total OPA1 expression (1.52; IQR 1.02–1.69 vs 0.67; IQR 0.54−0.88, p = 0.001) compared to those treated with Std-CPR (Fig. 3). OPA1 cleavage analysis showed higher OPA1 long to short chain ratio in animals treated with HD CPR compared to both animals treated with Std-CPR (0.63; IQR 0.46−0.92 vs 0.26; IQR 0.26−0.31, p = 0.016) and shams (0.63; IQR 0.46−0.92 vs 0.28; IQR 0.25–0.36, p = 0.025). Additionally, OPA1 cleavage analysis showed higher L-OPA1 (0.43; IQR 0.18−0.54 vs 0.06; IQR 0.05−0.08, p = 0.002) and S-OPA1 (0.56; IQR 0.40−0.64 vs 0.22; IQR 0.13−0.31, p = 0.003) in the HD-CPR arm compared to sham animals (Fig. 4). No significant effect was seen for MFN2 protein expression. There was also no significant effect on expression of total DRP1 or FIS1 between intervention cohorts (Fig. 3).
Fig. 3.
OPA1 protein expression is increased at 24 h after CA in animals receiving HD-CPR.
(A) Representative Western blot analysis of expression of fusion/fission proteins in homogenized cerebral cortex at 24 h in sham (n = 7), Std-CPR (n = 5) and HD-CPR (n = 5).
(B) Relative densitometry of OPA1 and MFN2 protein expression in relation to alpha tubulin loading control expressed as box plots. HD-CPR animals have increased OPA1 expression compared to sham (mean rank difference 10.4, p = 0.001).
(C) Relative densitometry of DRP1 and FIS1 protein expression in relation to alpha tubulin loading control expressed as box plots. No significant differences between groups.
Fig. 4.
OPA1 proteolytic cleavage products are increased in animals receiving HD-CPR versus shams. Ratio of L-OPA1 to S-OPA1 significantly increase at 24 h in HD-CPR animals.
(A) Western blot analysis of expression of OPA1 and cleavage products, L-OPA1 (111KDa), S-OPA1 (75−86kDA) in homogenized cerebral cortex at 24 h in sham (n = 7), Std-CPR (n = 5) and HD-CPR (n = 5).
(B) Relative densitometry of L-OPA1 and S-OPA1 normalized to alpha tubulin and L-OPA1/S-OPA1. L-OPA1 and S-OPA1 cleavage products are increased in HD-CPR versus sham (mean rank difference 10.0, p = 0.002 and mean rank difference 9.7, p = 0.003) respectively. L-OPA1/S-OPA1 ratio is greater in HD-CPR animals compared to Std-CPR (mean rank difference 8.8, p = 0.025) and sham (mean rank difference 7.8, p = 0.018).
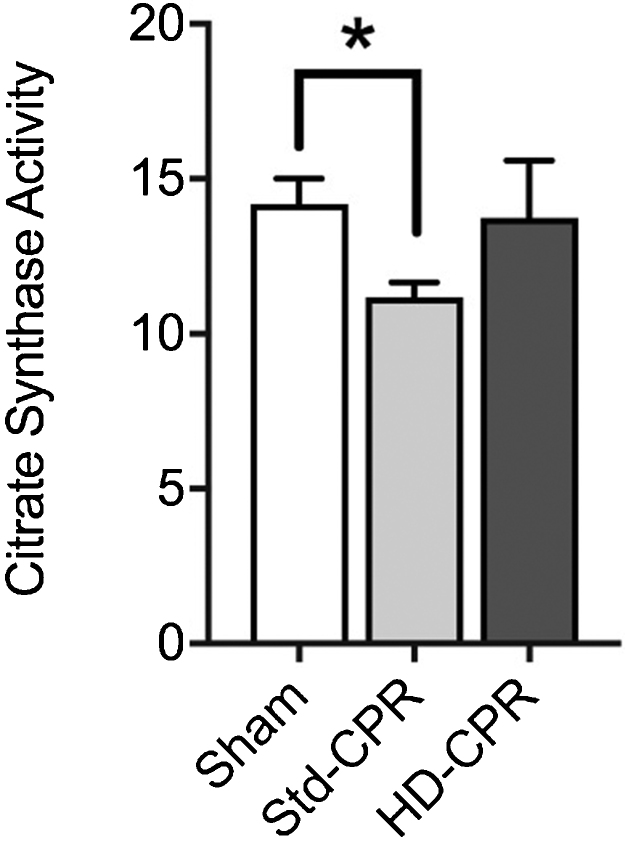
Mitochondrial mass
There was a decrease in mitochondrial mass in animals that were treated with Std-CPR compared to sham animals (11.0; IQR 10.15–12.29 vs 13.4; IQR 12.28–15.66, p = 0.047) and a preservation of mitochondrial mass in animals treated with HD-CPR compared to sham animals (Fig. 5). There was also a trend towards increased mitochondrial mass in the HD-CPR compared to Std-CPR (14.16; IQR 11.97–15.29 vs 11.02; IQR 10.15–12.29, p = 0.085).
Fig. 5.
Decreased mitochondrial mass in Std-CPR animals compared to sham animals.
Changes in citrate synthase activity (umol/mL/min) as a marker of mitochondrial mass in cerebral cortex homogenate in sham (n = 7), Std-CPR (n = 5) or HD-CPR (n = 5) 24 h after ROSC. Std-CPR animals have decreased mitochondrial mass compare to shams (mean rank difference 7.1, p = 0.047), with maintained mass in HD-CPR animals. HD-CPR animals with a trend towards greater mitochondrial mass compared to Std-CPR (mean rank difference 7.0, p = 0.085).
Porcine cerebral performance category
At 24 h after cardiac arrest, survivors of HD-CPR had normalization of CPC to shams, while Std-CPR animals had significantly worse neurologic scores than both animals that were treated with HD-CPR (3; IQR 2–3 vs 1; IQR 1–2, p = 0.014) and shams (3; IQR 2–3 vs 1; IQR 1−1, p = 0.0094) (Fig. 6).
Fig. 6.
Std-CPR (n = 5) had a higher porcine cerebral performance category indicating more neurologic dysfunction 24 h after CA compared to sham (n = 6, mean rank difference 6.4, p = 0.009) and HD-CPR (n = 5, mean rank difference 6.4, p = 0.014).
Discussion
Prior studies by our group comparing HD-CPR to Std-CPR in this model of pediatric asphyxia-associated cardiac arrest have demonstrated that HD-CPR results in improved mitochondrial respiration and higher rates of both ROSC and 24-h survival with favorable neurologic outcome.21, 22 The present study builds on this line of work by demonstrating that HD-CPR subjects had protein expression consistent with mitochondrial fusion in the cerebral cortex 24 h after cardiac arrest when compared to sham animals. This conclusion is supported by an upregulation of total OPA1 expression, increased OPA1 long to short chain ratio, and a preservation of mitochondrial mass. In contrast, compared to HD-CPR, Std-CPR resulted in decreased mitochondrial mass 24 h post-arrest, as measured by citrate synthase activity. This study also demonstrates improved intra-arrest haemodynamics and post-arrest neurologic scoring in subjects that received HD-CPR. Cumulatively, these data suggest that in subjects receiving HD-CPR, mitochondrial fusion may be driven by superior intra-arrest haemodynamics and represent an adaptive response that optimizes clinical neurologic recovery after asphyxial cardiac arrest.
Mitochondrial fusion is regulated by GTPase proteins optic atrophy 1 (OPA1) and mitofusins 1 and 2 (MFN1, MFN2). OPA1 exists in multiple isoforms; long isoform (L-OPA1) is embedded in the inner mitochondrial membrane and promotes mitochondrial fusion. A low ATP state or loss of mitochondrial potential can stimulate excessive OPA1 cleavage to short isoform (S-OPA1), which stimulates fission, increases mitochondrial membrane permeability, and promotes cell death.33 Mitochondrial fission is influenced by total expression and post-translational modifications of dynamin related protein (DRP1), a cytosolic GTPase that translocates to the mitochondrial outer membrane and binds to mitochondrial fission protein (FIS1).7, 8, 34 DRP1 inhibition with small molecule mdivi-1 is associated with improved survival and neurologic scores after ROSC in a murine model of cardiac arrest.9
Our findings are the first to document protein expression changes pertaining to mitochondrial dynamics in cerebral cortex following a cardiac arrest in a large animal model. To our knowledge, the only study of cerebral mitochondrial dynamics following ischaemia reperfusion injury was performed by Li et al. in an adult rodent asphyxial cardiac arrest model.10 Their study reported an upregulation of fusion proteins MFN1 and MFN2 at 4 h post-ROSC, followed by a rise in fission proteins DRP1 and FIS1 at 12−24 h. We found our most significant changes in OPA1 expression and OPA1 cleavage following cardiac arrest treated with HD-CPR, and did not detect differential expression of fission proteins DRP1 and FIS1. The differences in these findings may be attributed to the degree of injury (e.g., differences in injury model or resuscitation techniques), different protein expression in a higher order mammal that is more directly related to human response, or a difference in response to insult in the developing cortex versus an adult animal model.22, 35, 36
Additionally, in an adult rodent diffuse axonal injury traumatic brain injury (TBI) model, Di Petro et al. showed that moderate TBI yielded elevated OPA1 expression 48 h after injury, but an increased L-OPA1 to S-OPA1 ratio as early as 6 h after injury.16 Furthermore, rodents subjected to moderate TBI had preserved citrate synthase expression, also consistent with fusion. After severe TBI, differential cleavage yielded greater S-OPA1 versus L-OPA1 and an increase in DRP1 expression, consistent with mitochondrial fission.16 Their findings support an emerging paradigm of differential mitochondrial dynamics responses dictated by the severity of injury. During moderate stress or injury, mitochondrial fusion allows for maximal metabolic efficiency and redistribution of mitochondrial DNA, which is thought to be protective. However, after severe injury, a sustained energy deficit leads to fission, which precedes mitophagy and can lead to ROS generation and cell death.15, 16, 19, 37
Animals that were treated with HD-CPR had greater systemic blood pressure and cerebral perfusion pressure during cardiac arrest than those treated with Std-CPR. Presumably, this resulted in a less severe ischaemia reperfusion injury. Consistent with the paradigm that injury severity is an important determinant of mitochondrial dynamics, it appears that the less severe brain injury that occurred in HD-CPR was associated with a more pro-fusion state compared to Std-CPR. This result suggests that cerebral mitochondrial dynamics can be altered in cardiac arrest and potentially other forms of ischaemia reperfusion injury by optimizing brain perfusion and fuels speculation that pharmacologically targeting fusion could be beneficial.
Our findings supporting an environment of mitochondrial fusion in the cerebral cortex 24 h after HD-CPR in conjunction with our findings of improvements in haemodynamics and neurologic scoring, as well as previously reported improvements in bioenergetics and survivorship are consistent with evidence that mitochondrial fusion may be protective. In addition to indirect promotion of fusion by optimizing cerebral perfusion during cardiac arrest, directed therapies that manipulate mitochondrial dynamics will be an exciting future direction. Our findings in this study suggest that OPA1 expression and cleavage may be important in cerebral cortex after such an injury in the developing brain, and that further study into neurotherapeutics that promote fusion by targeting OPA1 expression and post-translational modification are justified. Mdivi1, an inhibitor of DRP1 translocation, has been shown in a rodent model to improve neurologic outcomes after cardiac arrest and warrants further study in a higher level animal model of cardiac arrest.9
Limitations
Alterations in mitochondrial dynamics found in this study are only in survivors at 24 h after cardiac arrest. Because of the significantly increased survival in subjects who received haemodynamic-directed CPR after cardiac arrest compared to guideline based CPR, the magnitude of difference in protein expression and mitochondrial mass may be less than what we would have found had all Std-CPR animals (presumably with greater injury) survived. Similarly, mitochondrial dynamics described after injury in other models have an evolving time course, which we were not able to assess in these experiments. Although DRP1 translocates to the mitochondrial membrane during fission, we did not study DRP1 expression in isolated mitochondria due to concern for a non-representative mitochondrial fraction. We also were not able to corroborate our protein expression findings with mitochondrial imaging (e.g., confocal or electron microscopy) or RNA expression because of experimental constraints. Future studies of the time course of proteomic, transcriptomic and histopathologic measures of mitochondrial dynamics with this highly relevant model will allow us to better define the injury and the optimal timing of therapeutic interventions. Finally, our findings were limited by a small sample size per group of piglets undergoing cardiac arrest and cardiopulmonary resuscitation.
Conclusion
We have demonstrated increased expression of proteins that promote mitochondrial fusion and preservation of mitochondrial mass in animals who received HD-CPR as compared to Std-CPR at 24 h after CA. This result is associated with higher intra-arrest haemodynamics parameters and superior post-arrest neurologic scoring, as well as previously described mitochondrial bioenergetics and survival. Further studies are warranted to define the time course and mechanisms of brain mitochondrial dynamics following cardiac arrest, and to interrogate therapeutics that target mitochondrial dynamics to confer neuroprotection and promote neurologic recovery following cardiac arrest.
Funding
This work was supported by the NIH National Institute of Child Health and Human DevelopmentF31HD085731 (Dr. Ko), NIH National Heart, Lung, and Blood InstituteT32HL007915 (Dr. Ko), NIH National Heart, Lung, and Blood Insitute K23HL148541 (Dr. Morgan), Tim Raymond Family Fund (Dr. Kilbaugh), NIH National Heart, Lung, and Blood Institute R01HL141386 (Dr. Kilbaugh).
Conflict of interest statement
The authors reported no other conflicts of interest specific to this manuscript.
CRediT authorship contribution statement
Kumaran Senthil: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing - original draft, Visualization. Ryan W. Morgan: Conceptualization, Methodology, Formal analysis, Investigation, Writing - review & editing, Supervision, Project administration. Marco M. Hefti: Formal analysis, Writing - review & editing. Michael Karlsson: Conceptualization, Methodology, Investigation, Writing - review & editing. Andrew J. Lautz: Conceptualization, Methodology, Investigation. Constantine D. Mavroudis: Methodology, Investigation. Tiffany Ko: Conceptualization, Methodology, Investigation, Funding acquisition. Vinay M. Nadkarni: Conceptualization, Methodology, Investigation, Resources, Writing - review & editing, Supervision. Johannes Ehinger: Conceptualization, Methodology, Writing - review & editing. Robert A. Berg: Conceptualization, Methodology, Investigation, Resources, Writing - review & editing, Supervision, Project administration. Robert M. Sutton: Conceptualization, Methodology, Investigation, Resources, Writing - review & editing, Supervision, Project administration. Francis X. McGowan: Conceptualization, Methodology, Formal analysis, Writing - review & editing, Supervision. Todd J. Kilbaugh: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Writing - review & editing, Supervision, Project administration, Funding acquisition.
References
- 1.Berg R.A., Sutton R.M., Holubkov R. Ratio of PICU versus ward cardiopulmonary resuscitation events is increasing. Crit Care Med. 2013;41:2292–2297. doi: 10.1097/CCM.0b013e31828cf0c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berg R.A., Nadkarni V.M., Clark A.E. Incidence and outcomes of cardiopulmonary resuscitation in PICUs. Crit Care Med. 2016;44:798–808. doi: 10.1097/CCM.0000000000001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holmberg M.J., Ross C.E., Fitzmaurice G.M. Annual incidence of adult and pediatric in-hospital cardiac arrest in the United States. Circ Cardiovasc Qual Outcomes. 2019;12:1–8. doi: 10.1161/circoutcomes.119.005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayoub I.M., Radhakrishnan J., Gazmuri R.J. Targeting mitochondria for resuscitation from cardiac arrest. Crit Care Med. 2008;36:S440–S446. doi: 10.1097/CCM.0b013e31818a89f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kilbaugh T.J., Sutton R.M., Karlsson M. Persistently altered brain mitochondrial bioenergetics after apparently successful resuscitation from cardiac arrest. J Am Heart Assoc. 2015;4:1–11. doi: 10.1161/JAHA.115.002232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gazmuri R.J., Radhakrishnan J. Protecting mitochondrial bioenergetic function during resuscitation from cardiac arrest. Crit Care Clin. 2012;28:245–270. doi: 10.1016/j.ccc.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharp W.W. Dynamin-related protein 1 as a therapeutic target in cardiac arrest. J Mol Med. 2015;93:243–252. doi: 10.1007/s00109-015-1257-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharp W.W., Archer S.L. Mitochondrial dynamics in cardiovascular disease: fission and fusion foretell form and function. J Mol Med. 2015;93:225–228. doi: 10.1007/s00109-015-1258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharp W.W., Beiser D.G., Fang Y.H. Inhibition of the mitochondrial fission protein dynamin-related protein 1 improves survival in a murine cardiac arrest model. Crit Care Med. 2015;43:e38–e47. doi: 10.1097/CCM.0000000000000817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y., Tang Q., Wang P. Dynamic changes of mitochondrial fusion and fission in brain injury after cardiac arrest in rats. BioMed Res Int. 2017;2017 doi: 10.1155/2017/1948070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rizzuto R., De Stefani D., Raffaello A., Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol. 2012;13:566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- 12.Sanderson T.H., Reynolds C.A., Kumar R., Przyklenk K., Hüttemann M. Molecular mechanisms of ischemia – reperfusion injury in brain: pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol Neurobiol. 2013:9–23. doi: 10.1007/s12035-012-8344-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X., Fang P., Mai J., Choi E.T., Wang H., Yang X. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J Hematol Oncol. 2013:1–19. doi: 10.1186/1756-8722-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vakifahmetoglu-Norberg H., Ouchida A.T., Norberg E. The role of mitochondria in metabolism and cell death. Biochem Biophys Res Commun. 2017;482:426–431. doi: 10.1016/j.bbrc.2016.11.088. [DOI] [PubMed] [Google Scholar]
- 15.Bohovych I., Chan S.S.L., Khalimonchuk O. Mitochondrial protein quality control: the mechanisms guarding mitochondrial health. Antioxid Redox Signal. 2015;22:977–994. doi: 10.1089/ars.2014.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Pietro V., Lazzarino G., Amorini A.M. Fusion or fission: The destiny of mitochondria in traumatic brain injury of different severities. Sci Rep. 2017;7:1–13. doi: 10.1038/s41598-017-09587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehmann S., Martins L.M. Insights into mitochondrial quality control pathways and Parkinson’s disease. J Mol Med. 2013;91:665–671. doi: 10.1007/s00109-013-1044-y. [DOI] [PubMed] [Google Scholar]
- 18.Youle R.J., Van Der Bliek A.M., Complementation F.P., Mitochondria B.D., Fusion M., Proteins F. REVIEW mitochondrial fission, fusion, and stress. Science (80-) 2012;337:1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rugarli E.I., Langer T. Mitochondrial quality control: a matter of life and death for neurons. EMBO J. 2012;31:1336–1349. doi: 10.1038/emboj.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Supinski G.S., Schroder E.A., Callahan L.A. Mitochondria and critical illness. Chest. 2019:1–13. doi: 10.1016/j.chest.2019.08.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan R.W., Kilbaugh T.J., Shoap W. A hemodynamic-directed approach to pediatric cardiopulmonary resuscitation (HD-CPR) improves survival. Resuscitation. 2017;111:41–47. doi: 10.1016/j.resuscitation.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lautz A.J., Morgan R.W., Karlsson M. Hemodynamic-directed cardiopulmonary resuscitation improves neurologic outcomes and mitochondrial function in the heart and brain. Crit Care Med. 2019;47:e241–e249. doi: 10.1097/CCM.0000000000003620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atkins D.L., Berger S., Duff J.P. Part 11: Pediatric basic life support and cardiopulmonary resuscitation quality: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132:S519–S525. doi: 10.1161/CIR.0000000000000265. [DOI] [PubMed] [Google Scholar]
- 24.Naim M.Y., Sutton R.M., Friess S.H. Blood pressure- and coronary perfusion pressure-targeted cardiopulmonary resuscitation improves 24-hour survival from ventricular fibrillation cardiac arrest. Crit Care Med. 2016:1–7. doi: 10.1097/CCM.0000000000001859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sutton R.M., Friess S.H., Naim M.Y. Patient-centric blood pressure-targeted cardiopulmonary resuscitation improves survival from cardiac arrest. Am J Respir Crit Care Med. 2014;190:1255–1262. doi: 10.1164/rccm.201407-1343OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neurauter A., Nysæther J., Kramer-Johansen J. Comparison of mechanical characteristics of the human and porcine chest during cardiopulmonary resuscitation. Resuscitation. 2009;80:463–469. doi: 10.1016/j.resuscitation.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Duhaime A.C., Margulies S.S., Durham S.R. Maturation-dependent response of the piglet brain to scaled cortical impact. J Neurosurg. 2000;93:455–462. doi: 10.3171/jns.2000.93.3.0455. [DOI] [PubMed] [Google Scholar]
- 28.Moler F.W., Meert K., Donaldson A.E. In-hospital versus out-of-hospital pediatric cardiac arrest: a multicenter cohort study. Crit Care Med. 2009;37:2259–2267. doi: 10.1097/CCM.0b013e3181a00a6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meert K.L., Donaldson A., Nadkarni V. Multicenter cohort study of in-hospital pediatric cardiac arrest. Pediatr Crit Care Med. 2009;10 doi: 10.1097/PCC.0b013e3181a7045c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilbaugh T.J., Karlsson M., Duhaime A.C., Hansson M.J., Elmer E., Margulies S.S. Mitochondrial response in a toddler-aged swine model following diffuse non-impact traumatic brain injury. Mitochondrion. 2016;26:19–25. doi: 10.1016/j.mito.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berg R.A., Chapman F.W., Berg M.D. Attenuated adult biphasic shocks compared with weight-based monophasic shocks in a swine model of prolonged pediatric ventricular fibrillation. Resuscitation. 2004;61:189–197. doi: 10.1016/j.resuscitation.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 32.Berg R.A., Sanders A.B., Kern K.B. Adverse hemodynamic effects of interrupting chest compressions for rescue breathing during cardiopulmonary resuscitation for ventricular fibrillation cardiac arrest. Circulation. 2001;104:2465–2470. doi: 10.1161/hc4501.098926. [DOI] [PubMed] [Google Scholar]
- 33.MacVicar T., Langer T. OPA1 processing in cell death and disease – the long and short of it. J Cell Sci. 2016;129:2297–2306. doi: 10.1242/jcs.159186. [DOI] [PubMed] [Google Scholar]
- 34.Archer S.L. Mitochondrial dynamics – mitochondrial fission and fusion in human diseases. N Engl J Med. 2013;369:2236–2251. doi: 10.1056/NEJMra1215233. [DOI] [PubMed] [Google Scholar]
- 35.Karlsson M., Pukenas B., Chawla S. Neuroprotective effects of cyclosporine in a porcine pre-clinical trial of focal traumatic brain injury. J Neurotrauma. 2019;36:14–24. doi: 10.1089/neu.2018.5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kilbaugh T.J., Bhandare S., Lorom D.H., Saraswati M., Robertson C.L., Margulies S.S. Cyclosporin a preserves mitochondrial function after traumatic brain injury in the immature rat and piglet. J Neurotrauma. 2011;28:763–774. doi: 10.1089/neu.2010.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Youle R., Van Der Bliek A. Mitochondrial fission, fusion, and stress. Science (80-) 2012;337:1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]