Fig. 2.
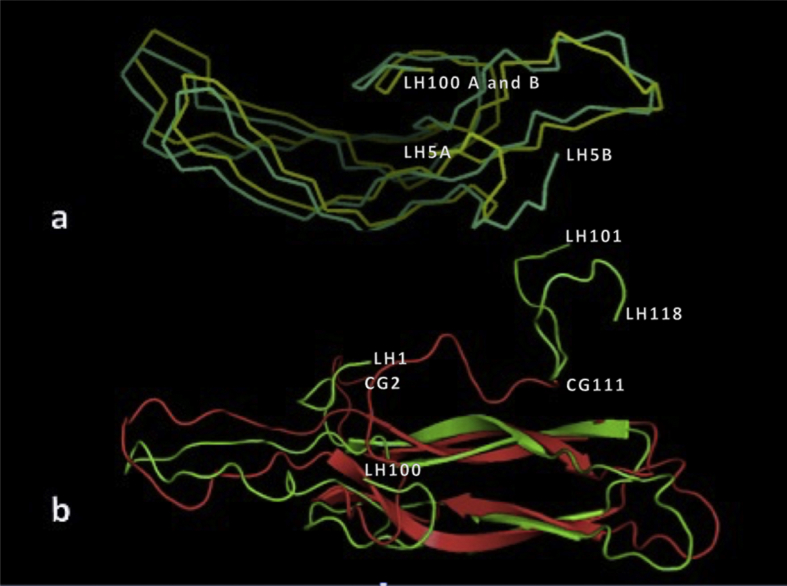
In (a), the Cα traces of two LH β subunits comprising the asymmetric unit of the crystals, in cyan and yellow, are superimposed showing the similarity of the two. Only main chain amino acids 5 through 100 were used for the superposition. The greatest differences between the two are at the amino and carboxy termini, and the segment 101 through 118, which was separated by proteolytic cleavage but remained linked to the body of the β subunit by the disulfide bond between cysteines 110 and 26. There are numerous differences in rotamer conformations between the two subunits as well. In (b) is shown the least squares superposition of a β subunit of luteinizing hormone in green upon the β subunit of human chorionic gonadotropin (from 1HCN). The similarity is strong in the cysteine – knot core, but it is evident that the extended, flexible loops diverge in conformation.