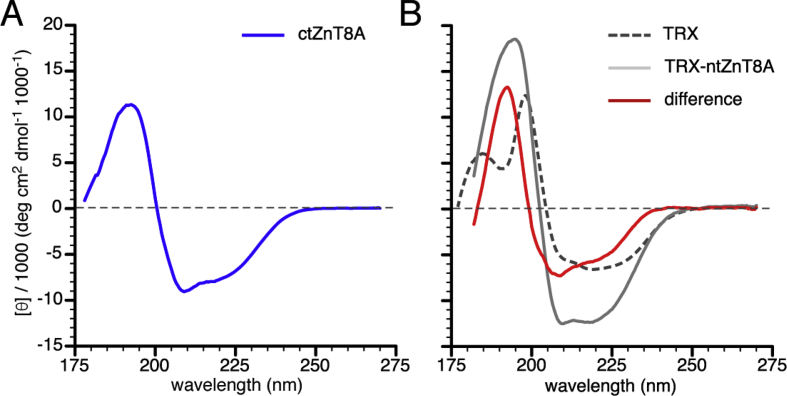
Fig. 5.
CD spectroscopy shows that the carboxy- and amino-terminal domains of ZnT8 show a mixed α-β fold. Far-ultraviolet CD spectrometry of ZnT8 termini and thioredoxin (TRX) constructs, showing molar ellipticity per residue versus wavelength. (A) Spectrum for the carboxy-terminal domain (ctZnT8). (B) Spectra for the amino-terminal domain (ntZnT8) constructs. ntZnT8-TRX and TRX spectra are shown in grey and stippled grey, respectively. The ntZnT8 difference spectrum (red line) was derived by subtracting the TRX spectrum from that of the ntZnT8-TRX fusion protein. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)