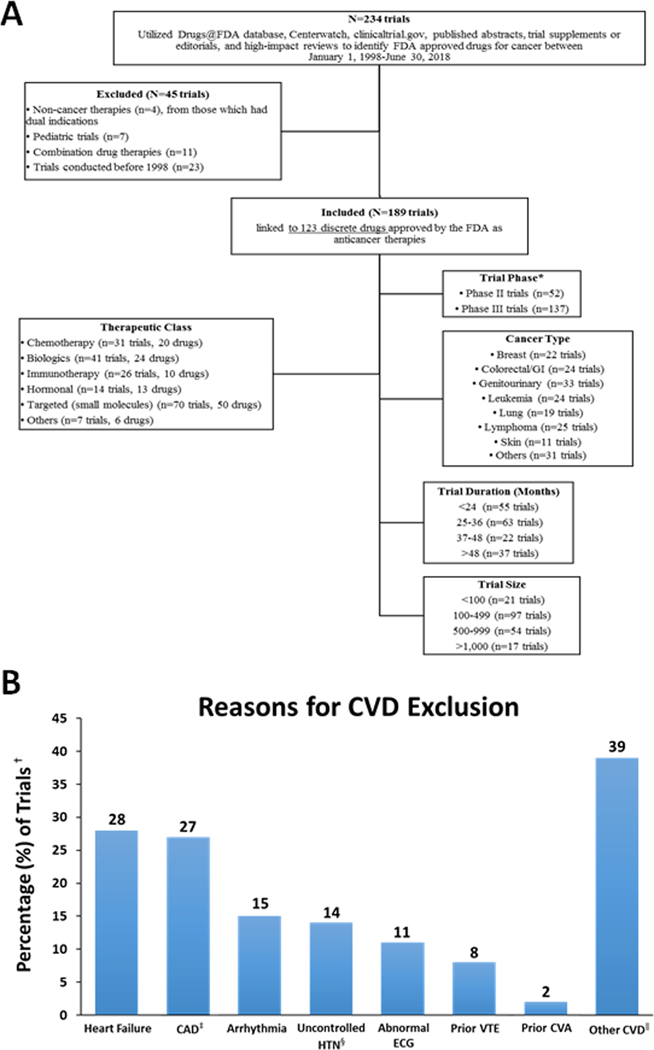
Figure 1.
Study consort diagram (A), and specific reasons for cardiovascular disease exclusion within pivotal cancer trials (B).
Abbreviations: BP, blood pressure; CAD, coronary artery disease; CVA, cerebrovascular accident; CVD, cardiovascular disease; ECG, electrocardiogram; HTN, hypertension; GI, gastrointestinal; VTE, venous thromboembolic disease.
*Several therapies were approved on the basis of breakthrough phase II data, with ongoing or as yet to be initiated phase III trials.
†The percentages reported reflect a denominator of 64 (the total number of trials with exclusion of cardiovascular disease patients).
‡CAD, includes myocardial infarction.
§Usually defined as >150/90 mmHg, consistent with mild (ie. stage 1) hypertension by the available contemporary guidelines.
∥Other CVD included valvular disease, myocarditis, or other mention of cardiovascular disease.