Fig. 2.
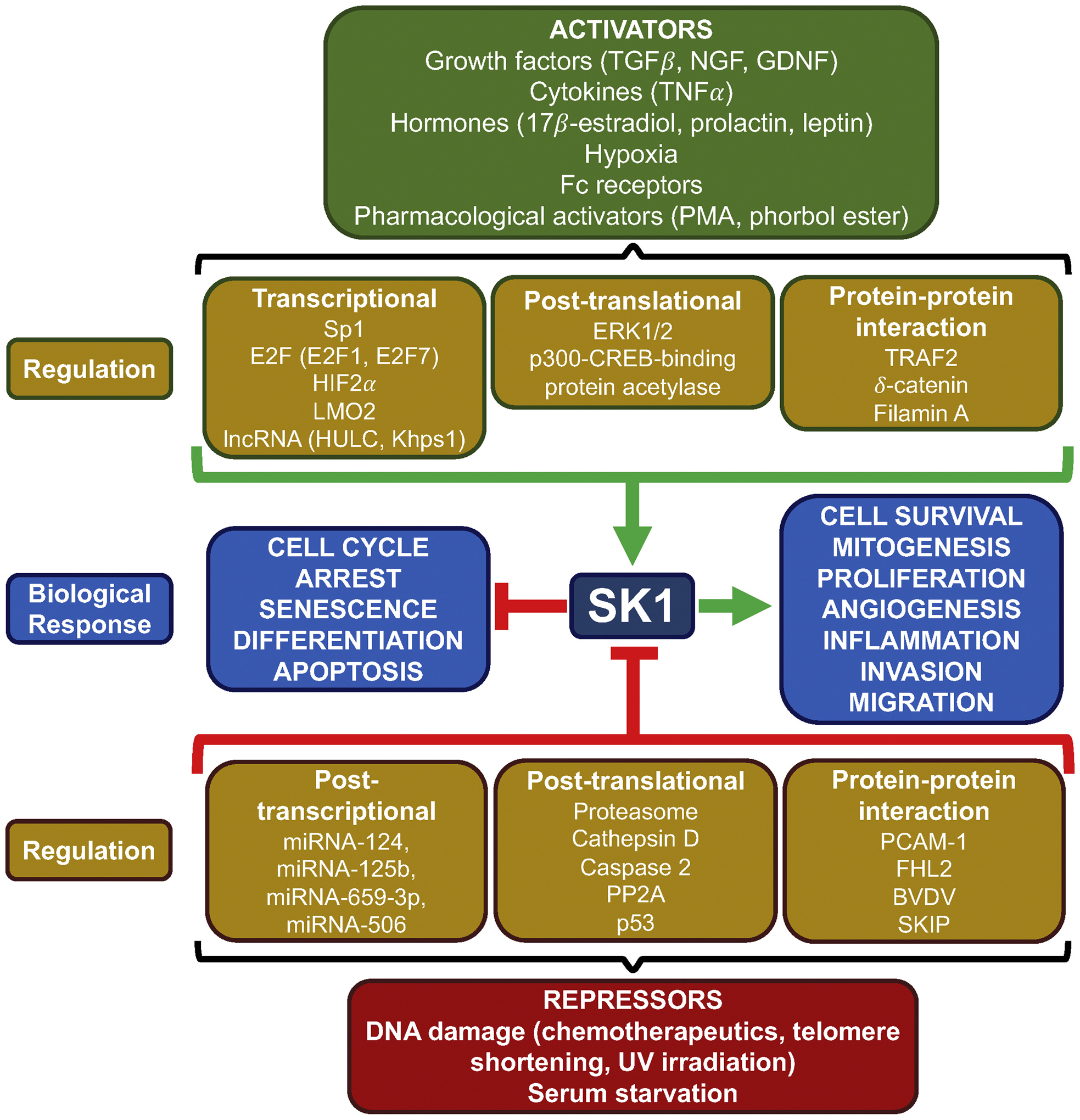
Regulation of SK1. SK1 is positively regulated by a wide range of signaling molecules including growth factors, cytokines and hormones as well as by pharmacological compounds, Fc receptors and hypoxia. These activators drive SK1 activity through various signaling pathways resulting in transcriptional upregulation, post-translational modifications including phosphorylation, or by protein-protein interactions. Increased SK1 activity is primarily associated with cell survival, mitogenesis, proliferation, angiogenesis, inflammation, invasion, or migration in a context-dependent manner. Alternatively, SK1 is negatively regulated by DNA damage induced by chemotherapeutics, UV irradiation, telomere shortening as well as serum starvation. Suppression of SK1 activity can be mediated through post-transcriptional repression by miRNA, post-translational proteasomal degradation, proteolysis via other proteases, dephosphorylation, or through interaction with proteins. Loss of SK1 activity is primarily associated with cell cycle arrest, senescence, apoptosis, and differentiation. PMA, phorbol 12-myristate 13-acetate; ERK, extracellular regulated kinase; TRAF2, TNF receptor-associated factor 2; PP2A, Protein phosphatase 2A; PECAM-1, platelet endothelial cell adhesion molecule 1; FHL2, four-and-a-half LIM domain 2; BVDV, bovine viral diarrhea virus.
