Figure 7. Working model of MR-dependent changes in baseline FKBP5 expression that modify GR sensitivity to glucocorticoids and subsequent HPA axis activity.
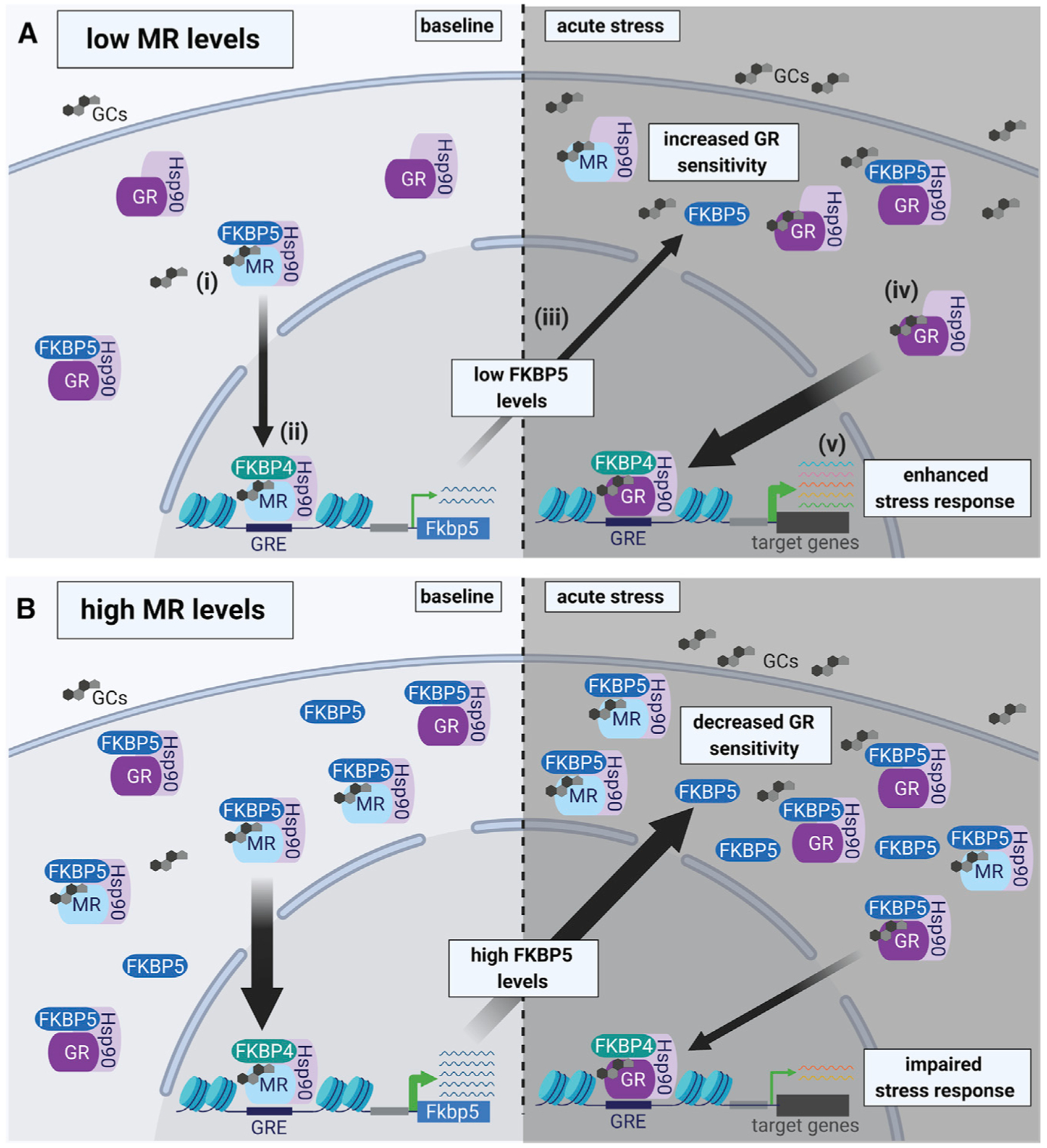
In the hippocampus MRs are largely occupied under basal glucocorticoid conditions, whereas GR occupancy is increased when glucocorticoid levels rise following acute stress. Upon glucocorticoid binding to the MR under baseline conditions (i), FKBP5 is replaced by FKBP4, which promotes the translocation of the MR-Hsp90 complex into the nucleus and subsequent DNA binding (to FKBP5 GREs) (ii). Thereby, the MR increases FKBP5 transcription and translation (iii), which can impact GR sensitivity (iv) and the subsequent stress response during acute stress (v).
(A) Low MR levels result in low FKBP5 expression under baseline conditions. In turn, low FKBP5 levels lead to increased GR sensitivity during acute stress, resulting in an enhanced stress response.
(B) In contrast, high levels of MR promote increased FKBP5 expression under baseline conditions. High levels of FKBP5 result in decreased GR sensitivity during acute stress, which leads to an impaired stress response. Of note, MRs and GRs can function either as homodimers or heterodimers (de Kloet et al., 2005; Mifsud and Reul, 2016). For simplicity, we did not include homodimers and heterodimers of corticosteroid receptors in this illustration. GCs, glucocorticoids; Hsp90, heat shock protein 90; FKBP4, FK506-binding protein 4.
