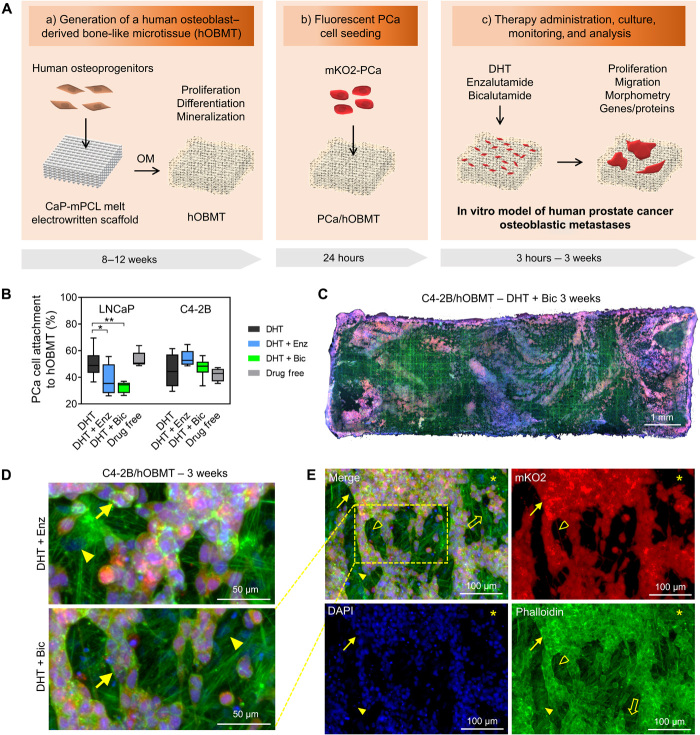
Fig. 1. Development of an in vitro microtissue-engineered 3D model of human prostate cancer osteoblastic metastases.
(A) Schematic overview of manufacturing involving (a) a calcium phosphate–coated melt electrowritten polycaprolactone (CaP-mPCL) scaffold seeded with primary human osteoprogenitors, followed by 8 to 12 weeks culture in osteogenic media (OM), leading to an hOBMT; (b) coculture with fluorescently tagged (mKO2) prostate cancer (PCa) cell lines for 24 hours; and (c) therapy administration, culture from 24 hours to 3 weeks, monitoring and analysis. (B) Attachment rates (%) of LNCaP and C4-2B cells to hOBMT conditioned in DHT (dihydrotestosterone), DHT + Enz (enzalutamide), DHT + Bic (bicalutamide), or drug-free for 7 days before coculture with hOBMT for 24 hours (average n = 5), *P < 0.05 and **P < 0.01. (C to E) Confocal microscopy images of the 3D metastatic microtissues after 3 weeks hOBMT coculture with C4-2B cells under DHT (10 nM) and bicalutamide (Bic, 10 μM) (C to E) or DHT + enzalutamide (Enz, 10 μM) (D) showing cancer cell coverage of hOBMT and formation of micrometastases (MaxProj. shown, 50-μm z-stacks). Split channels show C4-2B cells (mKO2 in red), cell nuclei [4′,6-diamidino-2-phenylindole (DAPI) in blue], and actin filaments (phalloidin in green). Asterisks (*) show hOBMT, full arrows show cancer cells, open arrows show scaffold fibers, full arrowheads show osteoblasts, and open arrowheads show a 70-μm-long cancer cell filopodia.