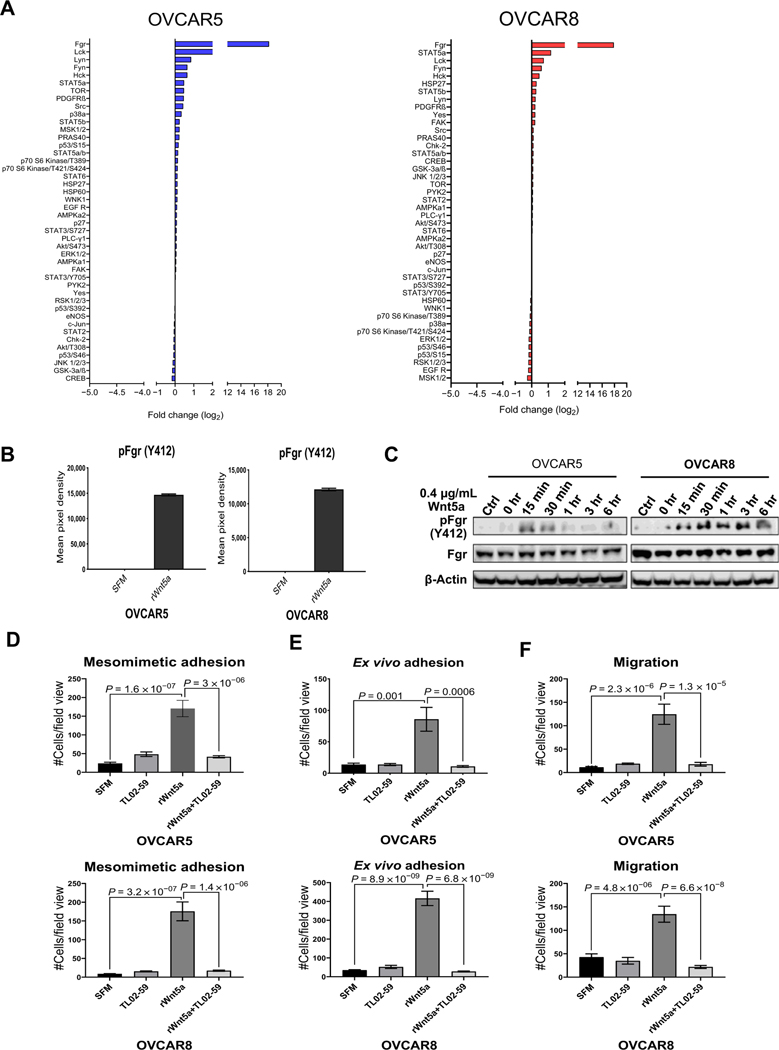
Figure 7.
Identification of Fgr as a downstream mediator of Wnt5a prometastatic cellular behavior. A, OVCAR5 and OVCAR8 cells were treated with SFM or rWnt5a protein (0.4 μg/mL, 24 hours) prior to lysis. Lysates were analyzed using the Proteome Profiler Human Phospho-Kinase Array according to the manufacturer’s specifications. Log2-fold change of phosphorylated proteins in the phosphokinase array is shown. B, Quantification of Fgr (Y412) phosphorylation by measuring pixel density for duplicate spots on the phospho-array blot using ImageJ. Data are presented as mean ± SEM. C, Validation of Fgr expression and phosphorylation. OVCAR5 and OVCAR8 cells were serum-starved for 2 hours then incubated with the Fgr inhibitor TL02–59 (0.01 μmol/L) for 2.5 hours. After washing, cells in SFM were incubated with rWnt5a protein (0.4 μg/mL) for the time points indicated. Ctrl, cells without rWnt5a treatment. Lysates were electrophoresed on SDS-polyacrylamide gels and immunoblotted with the antibodies noted. One representative blot from three independent biological replicates is shown. D and E, RFP-tagged OVCAR5 and OVCAR8 cells were treated with SFM, rWnt5a (0.4 mg/mL), rWnt5a (0.4 μg/mL), and the specific Fgr kinase inhibitor TL02–59 (1 μmol/L) or TL02–59 (1 μmol/L) for 24 hours, as indicated, prior to incubation with mesomimetic culture for 20 minutes or 1 hour (D), respectively, or with murine peritoneal explants in an ex vivo adhesion assay for 30 or 90 minutes (E), respectively. Quantification of adherent cells is shown for each condition. F, OVCAR5 and OVCAR8 cells were added to transwell migration chambers containing SFM, rWnt5a (0.4 μg/mL), rWnt5a (0.4 μg/mL), and TL02–59 (1 μmol/L), or TL02–59 (1 μmol/L) and incubated for 12 and 18 hours, respectively. Quantification of migrated cells in each condition.