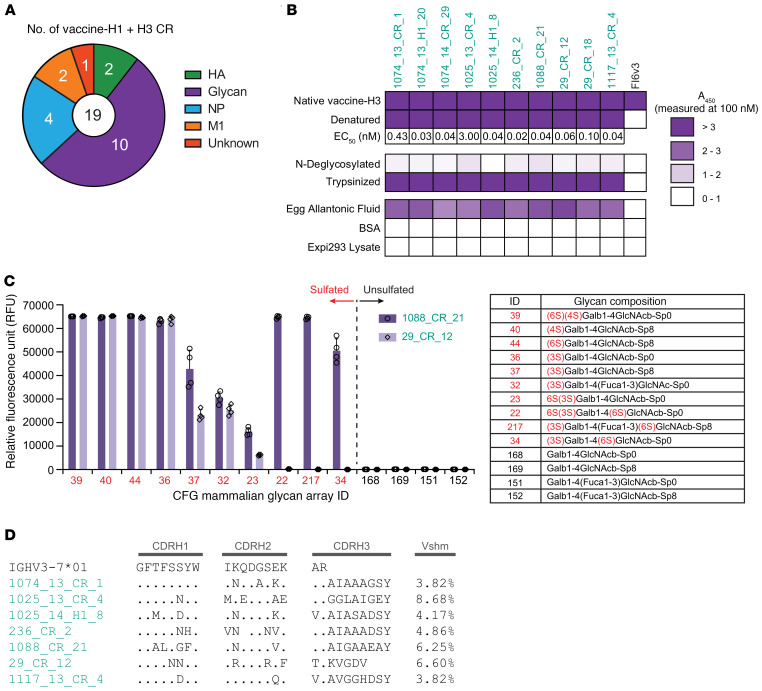
Figure 4. Characterization of s-mAbs that bind to sulfated glycans from egg components.
(A) Analysis showed that 17 of 19 vaccine-H1+H3 CR s-mAbs bound to components other than HA. (B) Binding profile of putative avian glycan–specific antibodies. ELISA assays were performed using 100 nM recombinant s-mAbs with their respective antigens. Denaturation was performed by boiling with SDS, and N-deglycosylation was from treatment with PNGase F after denaturation. Trypsinization was performed after reduction and alkylation. The EC50 was determined with denatured vaccine-H3 as the antigen. A450, absorbance at 450 nm. (C) Glycan array binding specificity of 2 s-mAbs with the glycan-targeting IGHV3–7 signature. RFU values for the top 10 glycans (red) and unsulfated versions (black) are shown with the mean ± SD for 4 replicates. (D) Sequence alignments of IGHV3–7 putative avian glycan–specific antibodies from different donors.