Abstract
Calvarial critical-size defects in rats are used to study regeneration of both craniofacial bone and long-bones. For decades, the trephine technique has been used with no notable refinements in the procedure. The use of piezoelectric surgical equipment has increased in human clinical oral and maxillofacial surgery, neurosurgery, traumatology, and orthopedics, because the devices are easy to handle, and can cut bone without damaging sensitive soft tissues such as blood vessels, nerves, and membranes. This study evaluated and compared the surgical technique and bone regeneration process between a traditional hand-drill trephine and piezoelectric equipment in a critical-size calvaria defect in a rat model. Thirty SD male rats were randomly divided into two groups and had either a 7.9mm diameter circular defect created with trephine or a 7.0mm square defect using the piezoelectric device, both creating 49 mm2 defect areas. MicroCT and histology were performed at 45 and 75d after surgery. While trephine surgeries were performed faster than piezoelectric (25.5 minutes vs 38.5 minutes), the rate of complications was much higher, with 36% of trephine rats taking 20 minutes to achieve hemostasis. Although the extent of new bone formation was similar between the two surgical groups, the piezoelectric technique resulted in 50% less variability. No additional new bone formation was observed from 45 to 75d in both techniques. Piezoelectric technique represents a refined and more reproducible technique for calvarial defect generation in comparison to classic trephine methods.
Keywords: Bone regeneration, Delayed union, Histology, Microcomputed tomography, Non-union, Parietal, Piezo, Skull, Ultrasonic
Introduction
Cranial defects can result from different pathologies such as congenital defects, trauma, tumor resection, decompressive intracranial procedures and secondary to resorbed bone flaps. Persistent calvarial defects can result in brain exposure to trauma, secondary neurological dysfunction and cosmetic morbidity (Chang, 2010; Xu, 2015; Williams, 2016; Honeybul, 2017, Piazza, 2017). The surgical repair of a skull defect regardless of its etiology is called cranioplasty (Hill, 2012).
Cranioplasty has been used for many years, but new surgical techniques and new materials to fill defects are continually being developed or improved to treat this complex problem (Bonfield, 2014; Piazza, 2017). Biomaterials in current use in human clinical cases include acrylics, ceramics, polyethene, polyetheretherketone (PEEK) and titanium (Williams, 2016). In addition to these treatments for clinical bone defects, new bone regeneration therapies have been tested in pre-clinical stages to reconstruct calvaria defects in animal models with the aim to promote brain protection and restore a more normal appearance (Dumanian, 2017; Wongsupa, 2017; Youngstrom, 2017). In this way, calvaria critical-size defects in animal models are useful to provide a proof-of-principle to develop therapies for more effective bone repair (Gomes, 2011). The term ‘critical-sized defect’ is used to describe a defect that will not heal spontaneously during the period of an experiment (Gosain, 2000). The rat calvarial defect model is one of the most widely used models to study bone regeneration for both craniofacial and long bone repair, with 100s of examples in the literature. Critical-size defects in rat are usually created using trephines of different diameter size, from 5.0 to 9.0mm, on parietal bones, bilateral or centralized (Vajgel, 2014). Although those techniques are straightforward, adverse events are reported, including, bone necrosis due to the generation of heat during the osteotomy and dura mater damage (Greenwald, 2000; Sawyer, 2009). It is important to investigate alternative technique that are responsive to the 3R’s principle (reduction, refinement and replacement), to produce more reproducible results with less deviation and enhanced animal welfare (Vajgel, 2014).
Piezoelectric ultrasonic technology has been extensively used in oral and maxillofacial surgery, neurosurgery, traumatology, and orthopedics (Robiony, 2004). One advantage of piezoelectric technology is the selective effect on mineralized tissues, while avoiding damage to sensitive structures and soft tissues such as blood vessels, nerves, and membranes, such as the dura mater (Kerawala, 1999; Landes, 2008). Previous studies have shown that for the surgeon using piezoelectric ultrasonic equipment permits more precise cuts than rotary hand pieces (Horton, 1981). In addition, morphological evaluations have shown no signs of necrosis or pigmentation on piezoeletric studies, which could represent benefits for bone healing in comparison to other classic trephine techniques that generate heat mechanically during the osteotomy (Robiony, 2004; Sawyer, 2009).
The purpose of the present study was to evaluate and compare the time of surgery, intra-operative complications, post-operative outcome, and bone regeneration in the generation of rat critical-size calvaria defects with piezoelectric technique vs traditional trephine.
Materials and Methods
Ethical aspects, animals and conditions
Experimental procedures were consistent with ethical principles for animal research and were approved by both University of Michigan and Michigan State University IACUC.
Thirty adult male Sprague-Dawley rats (Rattus norvegicus), 5–6 months of age, and 450–520g (due to parietal bones minimum size), were used. Throughout the study, rats were housed in pairs in polypropylene cages (37 cm x 25 cm x 24 cm), with 12/12 night/day cycles, 21°C (± 2°C) and 50% (± 20%) relative humidity. All rats had ad libitum access to complete rat chow and filtered water.
Critical-sized healing defect model and experimental design
Rats were randomly assigned to two surgical approach groups: 1) 7.9mm diameter circular defect using trephine – Circular group, 16 rats; or 2) 7.0mm side square defect using piezoelectric equipment – Square group, 14 rats. The size of the circular defect was chosen based on a survey of the literature for a trephine technique that produces non-union (Vajgel, 2014). Then, a comparable square size was calculated (s2 = πr2; which “s” represents square side and “r” represents the ray of the circumference) to produce the same size defect area in both models. Calvaria were harvested at three-time points for each group: 1) day zero, surgical technique control performed in 2 rats per group after euthanasia; 2) 45 days after surgery, 6 rats per group; 3) 75 days after surgery, 6 rats per group. Two rats of Circle group were excluded from analysis due to immediate post-operative death.
Anesthesia was induced using isoflurane at 3.5%, and rats were maintained at 2–2.5%. Pain relief was provided with a single dose of buprenorphine SR (1mg/kg SC) just after the anesthetic induction.
All instruments were sterilized, surgical table and environment were kept in aseptic conditions, sterilized disposable gowns, gloves and drapes were used. The dorsum of the rats was shaved from the eyes line to the 1/3 cranial neck, including the areas surrounding the ears. The surgical field was cleaned with chlorhexidine and ethanol 70% using 3 repeating washes. During the procedure, animals were maintained in ventral recumbence.
Square defect surgical technique using piezoelectric equipment (Fig. 1)
Fig. 1:

Operative technique to produce the 7.0mm sided square critical-size defect in rat calvaria using piezoelectric equipment. A) surgical field preparation; B) sagittal skin incision; C) periosteum exposure after dissection of subcutaneous tissue; D) periosteum removal using piezoelectric equipment; E) parietal bones exposure; F) square template to perform the osteotomy; G) osteotomy using the piezoelectric equipment; H) segmental loose bone after osteotomy in square shape; I) 7.0mm sided square critical-size defect; J) skin suture.
A 3 – 3.5cm sagittal skin incision was made from 0.5–1.0cm caudal to a mid-line drawn between the eyes to direction of neck;
Subcutaneous tissue was dissected producing a clear view from the left temporal muscle to the right temporal muscle;
Periosteum was incised parallel to one of the temporal muscle with the scalpel;
Using the Piezosurgery® GP equipment and PR1 insert (Mectron Medical Technology, Carasco, Italy) in the Endo function and irrigation level 1 (sterilized distilled water), the periosteum was totally removed from the parietal bones until Temporal muscles level;
Using ruler and pencil, a 7.0mm side square was drawn onto the parietal bones, using the Sagittal suture as the midline reference.
Osteotomy of the parietal bones was completed using the Piezosurgery® GP equipment and OT1 insert (Mectron) in cortical function and irrigation level1 (sterilized distilled water);
Segmental bone in the defect after the osteotomy was removed using the scalpel;
Skin was sutured using 4–0 nylon suture.
Circular defect surgical technique using trephine (Fig. 2)
Fig. 2:

7.9mm diameter circular critical-size defect technique using trephine. A) Osteotomy of parietal bones using hand-drill along with trephine; B) Circular critical-size defect after osteotomy.
Surgical site preparation was similar to that described for the Square defect;
A 3 – 3.5cm sagittal skin incision was made from 0.5–1.0cm caudal to a line of the eyes to direction of neck;
Subcutaneous tissue was dissected until a clear view was produced from the left temporal muscle to the right temporal muscle;
Periosteum was mechanically removed by rubbing the periosteum from the parietal bones using the scalpel;
The osteotomy was done using unidirectional rotational movements with a hand-drill (WowParts®) attached to a titanium alloy 7.9mm diameter (outer) trephine (Ace Surgical Supply®). Irrigation was constant during the osteotomy using sterilized distilled water.
The freed bone in the defect after the osteotomy was removed using the scalpel;
Skin was sutured in a using 4–0 nylon suture, as described.
Surgical general notes
Surgical procedures were timed from the first incision to last suture on the first 12 live animals of each group. Transoperational technical difficulties and post operatory recovery were documented.
Euthanasia and sample collection
The rats were euthanized with a CO2 overdose based on the AVMA standards for euthanasia. Calvaria bones of both Square and Circular group were harvested using a Piezoeletric equipment (Piezosurgery® GP and OT12 insert, Mectron) using the interparietal bone as caudal board, the frontal bones as rostral boards and temporal bones as lateral boards. Bones were fixed in 10% buffered formalin.
Micro-computed tomography (microCT)
General morphological description and morphometric analysis were assessed using microCT. MicroCT analysis was performed using a Skyscan 1076 scanner (Bruker-MicroCT, Kontich, Belgium). Ex-vivo specimens were immersed in water and scanned at 65KV, 381μA, 1mm aluminum filter in 180 degrees, two frames per 0.3°, 9μm voxel size, 200ms. Images were reconstructed using NRecon® Reconstruction software images in “Hounsfield Unit” (HU), smoothing 3.0, 2.0 misalignement compensation, ring artifact reduction 10, 30% of beam-hardening correction; the same contrast and brightness was used for all samples. DataViewer software was used to re-align the images and quantitative parameters were assessed using Skyscan CTan software (SkyScan,Kontich, Belgium). Bone voxels were determined with a global threshold of 63mm minimum and 255mm maximum, which was selected considering the average minimum auto-threshold for each file. Bone area (BAr) 2D analysis was done using two different region of interests (ROI’s): 1) a 7.0mm x 7.0mm ROI in Square group; and 2) 7.9mm diameter in the Circle group. In both cases we determined the slice subjectively that presented the most bone formation in each sample. Ratio Bone Volume per Tissue Volume (BV/TV) 3D analysis (bone volume relative to total volume – BV/TV) was done on two different volume of interests (VOI’s): 1) 7.0mm (l) x 7.0mm (w) x 0.25mm (h) in the defect site to generate a BV/TV of the Square group, and 2) 7.9mm diameter x 0.25mm thickness for CG. 3D images were generated using Skyscan CTAn.
Histological analysis
Each calvaria was sectioned in the median plane and the left halves were processed according to routine histological methods, including 10% EDTA, pH 7.0 decalcification, and embedded individually in Paraplast®. From each paraffin-embedded sample, 5μm thick sagittal sections were stained with hematoxylin and eosin. The analyzed fields of each section were then digitized with final magnification of 100x and 40x by a light microscopy Nikon® Eclipse Ni along with a Nikon® DS-Ri2 camera (Nikon Instruments Inc., Melville, NY, USA). Pictures and analysis were done using NIS-Elements BR 4.30.02–64 bit software (Nikon Instruments Inc., Melville, NY, USA).
Statistical analysis
Mean, standard deviation and Student’s t-test were used to compare the time taken to perform Square and Circular surgical technique. Mean, standard deviation and Mann-Whitney (Test U) was applied to compare microCT results in Square and Circular and to compare 45d and 75d of each group using Real Statistics Resource Pack software (Release 6.8; Copyright 2013 – 2020, Charles Zaiontz; www.real-statistics.com).
Graphs were generated using GraphPad Prism software version 8.0.0 for Windows (GraphPad Software, San Diego, USA, www.graphpad.com).
Results
Surgical observations
Two rats of the Circular group (14%) died in the first two hours during post-operative care. While the cause of death was not confirmed by post-mortem exam, this likely occurred because they did not recover from extensive trans operative bleeding. There was extensive hemorrhage in all animals of the Circular group; in 5 cases (36%), more than 20 minutes was required to achieve hemostasis by compression after trephine calvaria osteotomies. No complications were registered in Square group.
Significant differences (p=0.004) were reported on the time to perform each type of surgical technique. Circular group mean surgical time was 25.5 minutes (± 4.5) from first incision to last suture, while Square group mean was 38.5 minutes (± 9.4).
Following the post-operative recovery period from day 2 until day 75, no behavioral differences were noticed of either group of rats.
Micro-computed tomography (microCT)
Sequential 2D slices and 3D reconstructions showed new bone mass in both Square and Circular group of 45d and 75d in the margin of the calvaria defects (Fig. 3). In three of the 14 samples (21%) of Circular group, the defect was not constrained to temporal bones, but also involved the adjacent bones (frontal and interparietal) (Fig. 4A).
Fig. 3:

Square and circular defects show similar extent of regeneration. Representative microCT reconstructions of bone regeneration in 7.0mm sided square critical-size defect created using piezoelectric equipment and 7.9mm diameter circular critical-size defect using trephine. The red line in 3D dorsal views indicates the 2D transverse sections points below, each dorsal projection.
Fig. 4:
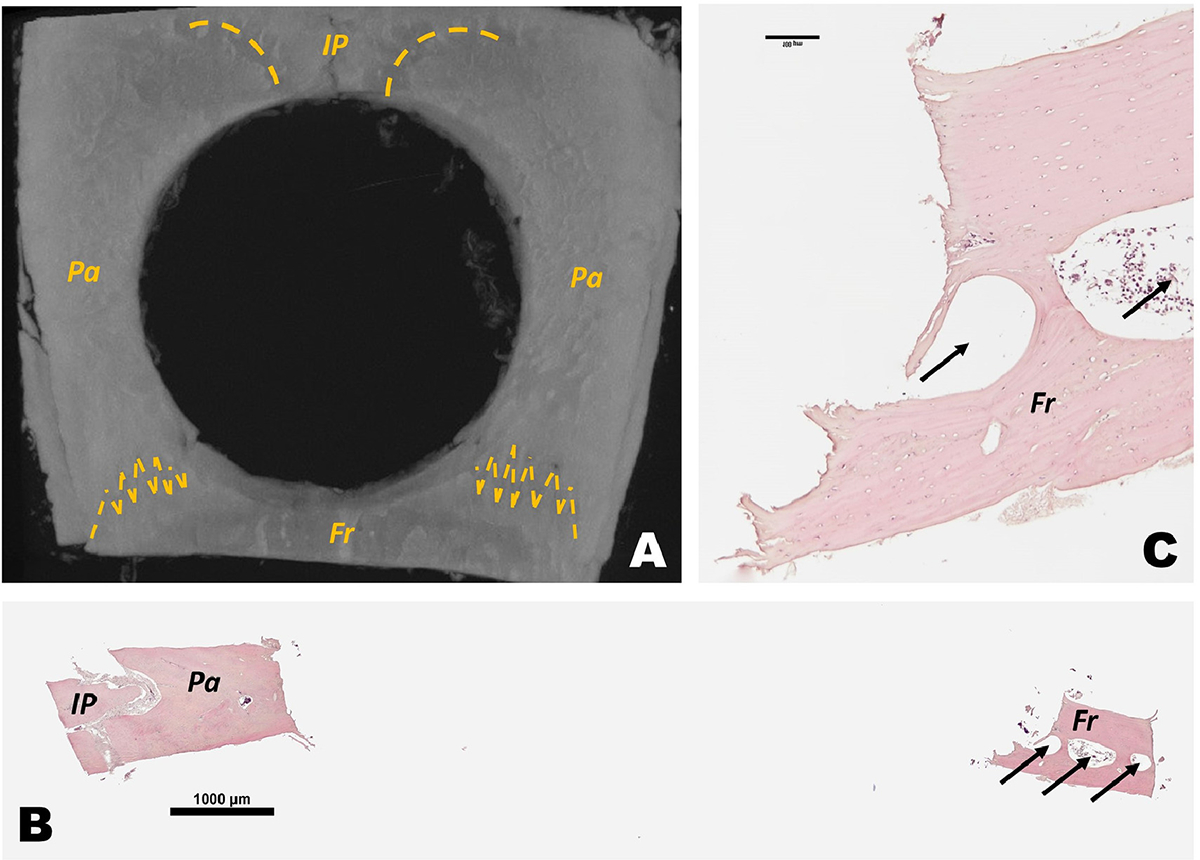
Circular critical-sized defect invades frontal and interparietal bone. A) Representative microCT 3D reconstruction of rat calvaria with 7.9mm diameter segmental defect in parietal bones also impacts interparietal and frontal bone at day 0. Yellow dash lines marks the suture between bones. B) H&E histological scan of sagittal sections of circular segmental defect. C) magnified H&E histological sagittal section of frontal bone in a circular segmental defect in calvaria. IP = interparietal bones; Pa = parietal bone; Fr = frontal bones. Arrows indicate frontal bone sinus.
Statistical comparison between Square and Circular groups did not show significant differences for 3D BV/TV nor 2D BAr at 45d (p=0.12 and p=0.17, respectively) and 75d (p=0.40 and p=0.31, respectively. Also, there was no statistically significant differences in Square group new bone formation from 45d to 75d for 2D BAr (p=0.23) and 3D BV/TV (p=0.12). Similarly for the Circular group, statistical analysis showed no significant differences between 45d and 75d for 2D BAr (p=0.40) and 3D BV/TV (p=0.31). While new bone formed in both Circular and Square groups at 45d and 75d for 3D BV/TV and 2D BAr values were similar, the Circular group had higher standard deviation at both 45d and 75d time points (Table 1; Fig. 5).
Table 1:
Average values and standard deviation (SD) of 2D and 3D microCT analysis of bone regeneration of parietal bones in 7.9mm diameter circular (C) critical-size defect and 7.0 mm sided square (Sq) critical-size defect at 0, 45 and 75days after surgery.
| 2D Measurements | 3D Measurements | ||||
|---|---|---|---|---|---|
| Time point | TS [mm2] | Bar (SD) [mm2] | TV [mm3] | BV/TV (SD) [%] | |
| Circular (Trephine) | 0d | 49 | 0.01 (0.00) | 12 | 0.01 (0.00) |
| 45d | 49 | 9.64 (3.97) | 12 | 18.67 (7.34) | |
| 75d | 49 | 7.74 (5.42) | 12 | 16.00 (10.59) | |
| Square (Piezosurgery) | 0d | 49 | 0.09 (0.01) | 12 | 0.22 (0.04) |
| 45d | 49 | 11.75 (3.75) | 12 | 14.37 (6.11) | |
| 75d | 49 | 9.00 (2.57) | 12 | 16.27 (5.21) | |
Notes: TS = tissue surface, Bar = bone area, TV = tissue volume, BV/TV = bone volume per tissue volume.
Fig. 5:

Distribution of bone regeneration in the defects of each group analyzed in microCT. Bone volume per tissue volume (BV/TV) and area of bone formation (BAr) did not indicate statistical significance (p < 0.05) between Circular (trephine) and Square (piezoelectric) groups in all time points; nor between 45-days and 75-days groups. Zero-day (control) groups showed statistical differences in comparison to 45-days and 75-days in both Circular and Square techniques in the analyzed parameters.
Histological analysis
Sagittal sections stained with H&E revealed some new bone formation comparing 0d to 45 and 75d in both Circular and Square group (Fig. 6). As well, the defects were filled with non-bone connective tissue. The connective tissue formed in calvarial defects of both the 45d and 75d Circle defects was much thicker than Square group at 45d and 75d. However, in both groups, there was a reduction of the same connective tissue thickness from 45d to 75d (Fig. 6). There was no evidence of bone formation processes bridging the defects in either the Circular or Square groups at 45d and 75d. The new bone was limited to the margin of the segmental defect (Fig. 6). Some sections of the Circle group showed the defect extended to outside of the parietal bone to adjacent bones and reached the frontal bone sinus (Fig. 4B–C).
Fig. 6:
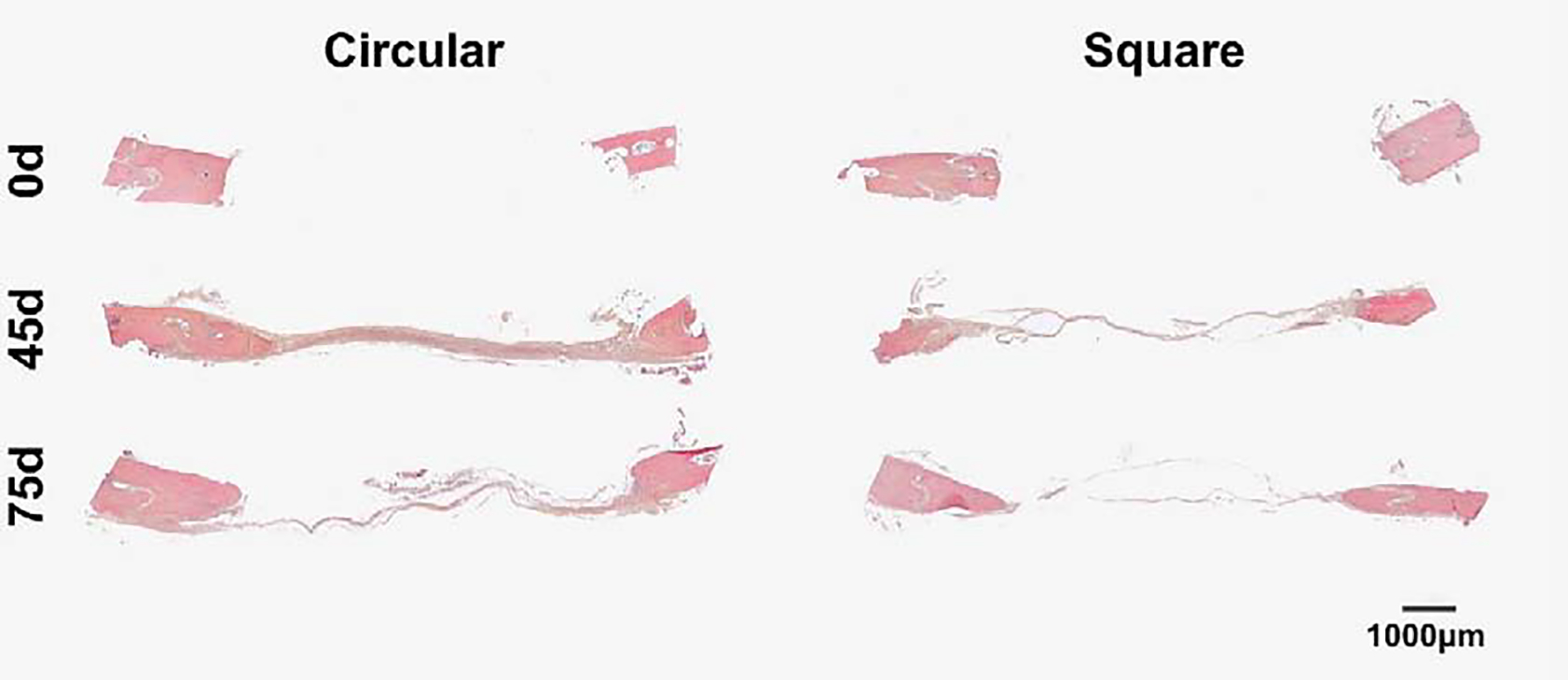
Circular defects show thicker connective tissue at both 45d and 75d. Comparative representative sagittal histological images of rat calvaria of Circular and Square group at 0d, 45d and 75d after osteotomy, stained with H&E. Differences between groups and time points on the thickness and absence of connective tissue at segment defect. 40x scan.
Discussion
Creating square defects using the piezoelectric technique was safer than creating circular defects using trephine. Two out of 16 rats died during postoperative care and hemorrhage complications during trephine surgeries contrasts with the absence of notable complications in the Square group. Potential adverse consequences for trephine treated animals are not widely reported in previous studies, despite known dura mater damage (Greenwald, 2000; Vajgel, 2014). For decades, critical-size defects in calvaria of rats were done using trephine with few technical refinements (Takagi, 1982; Schmitz, 1990; Pryor, 2005; Fabris, 2018). The use of the piezoelectric technique is an advance with respect to animal welfare.
The trephine technique was performed faster than piezoelectric technique. Changing of the piezoeletric equipment inserts and cutting geometrical square in the Square group demanded significantly more time than the osteotomy using a circular trephine in Circular group. There are many inserts for the piezoeletric equipment, and it may be that using different inserts could result in appreciable time savings, as has been shown in other osteotomy technique in humans (Pagotto, 2017), but we used a standard insert throughout our experiment. It is notable that human clinical trials comparing piezoeletric devices to traditional methods have similarly show increased surgical time with piezoeletric devices (Rana 2013; Spinelli 2014).
Regarding bone regeneration, although the Circular and Square groups presented similar extent of new bone formation in microCT and histology, the circle group showed greater variability relative to the Square group, especially at day 75. The standard deviation was more than two-fold that of the Square. This is important because it suggests that using the piezoelectric equipment could result in a reduction in animal numbers required to achieve statistical significance in experimental studies, if there is less data variability. Additionally, in the circle group 450–520g rats may not have parietal bones large enough to permit the creation of 7.9mm circular defects to reach 49mm2 defect area. MicroCT and histology showed that the trephine osteotomy crossed the parietal and frontal sutures, invaded interparietal and frontal bones, which did not happen in the square shape that demanded shorter lines (7.0mm) to reach the same defect area. Invading adjacent bones in the circular defect may further compromise the results because of different regeneration processes between different calvarial bones and due to the presence of bone sinus and skull suture tissue. Invading adjacent bones in the circular defect may further compromise results because of different regeneration processes between different calvarial bones and due to the presence of bone sinus and skull suture tissue. The presented results are in agreement with variability assessed in other osteotomy and bone regeneration pre-clinical studies that compare piezoelectric and classic techniques in rats and rabbits, in tibia and mandible, respectfully (Esteves, 2013; Tosun, 2017). Our results represent that piezoelectric technique may not only be used as alternative model, but also it is more reproducible for studies on cranioplasty.
The piezoelectric technique was able to produce bone critical-sized defect non-unions in calvaria, similar to that of the traditional circular trephine. This was important to verify since the reduced soft-tissue trauma could have altered the extent of healing with the piezoeletric device. Since there were not significant differences in the extent of new bone regeneration from 45 to 75d in both groups using microCT, and histological analysis did not show new bone formation at the osteotomy edges at both harvest points, it is presumed that the bone defects of 49 mm2 do not heal after 45d in rat parietal bones – independent of the geometry of the defect or osteotomy method. In comparison to other craniotomies studies performed with 8.0mm trephine in rats, the presented results indicated less regeneration than the Stephan et al study (2010), possibly because periosteum was removed in our study to avoid technique bias on periosteum preservation between the methods. However, other published studies have shown that bone regeneration at different timepoints in calvaria has even lower bone regeneration rates (Vajgel, 2014).
While the focus of our work was on refining a preclinical model, it is notable that piezoelectric surgery is used clinically in human surgery for both the craniofacial skeleton and long bones (Gonzalez-Lagunas 2017, Russo, 2019). Specifically, while studies show that piezoelectric-based surgeries do take a longer time to complete, there is reduced soft-tissue trauma and operative bleeding. Indeed, groups report quantitative measures of reduced soft-tissue trauma, including reduced nerve damage in orthognathic surgery (Spinelli, 2014). Thus, our work may not only have implications for studying bone regeneration therapeutics in pre-clinical models, but it may also be useful for continuing to advance piezoeletric surgical approaches for human surgeries.
Conclusion
Both Circular (created with trephine) and Square (created with piezoelectric device) models show that the minimal regeneration process that occurs at the edges of the osteotomy in both approaches does not advance after 45d, which results in non-union. Critical-size defect in calvaria using piezoelectric device produced similar bone regeneration results to the standard trephine approach, but with better reproducibility (reduced standard deviation). In addition, piezoelectric technique was safer and caused less soft-tissue damage during the trans and post-operative care than trephine, which showed extensive bleeding and mortality. Although, there is still an opportunity to reduce the time of surgery using the piezoelectric technique with the introduction of new inserts or reduction of the size of the defect, the proposed model represents a refinement in calvaria critical-size defect using rats. Based on the advantages we evaluated in this large defect model, piezoelectric surgery could also refine smaller defect approaches, such as 3.0mm bilateral defect in rodents.
Supplementary Material
Highlights.
Creating large segmental defect in rat calvaria using Piezosurgery is safer than trephine method;
There is no increase of bone formation rate on calvaria defect (both in Piezo and trephine technique) after 45 days;
The bone regeneration rate using Piezosurgery technique to produce segmental defect is equivalent to trephine technique;
Piezosurgery technique is more reproducible than trephine, due to low standard deviation results in new bone formation;
Trephine technique is faster to perform than Piezosurgery technique.
Acknowledgements
We thank MS Kaela Navarro and Veterinary Technologist Heather Defoe for technical support during surgeries and microCT analysis. This work was in part supported by funding from the Congressional Directed Medical Research Program (CDMRP) Peer Reviewed Orthopaedic Research Program (PRORP) (OR140396). Research reported in this publication was also supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health (NIH) under Award Number P30 AR069620. The content is solely the responsibility of the authors and does not necessarily represent the official views of the CDMRP nor NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Bonfield CM, Kumar AR, Gerszten PC. The history of military cranioplasty. Neurosurg Focus 2014;36(4):E18. [DOI] [PubMed] [Google Scholar]
- Chang KP, Lai CH, Chang CH, Lin CL, Lai CS, Lin SD. Free flap options for reconstruction of complicated scalp and calvarial defects: report of a series of cases and literature review. Microsurg 2010;30(1):13–8. [DOI] [PubMed] [Google Scholar]
- Dumanian ZP, Tollemar V, Ye J, Lu M, Zhu Y, Liao J, Ameer GA, He TC, Reid RR. Repair of critical sized cranial defects with BMP9-transduced calvarial cells delivered in a thermoresponsive scaffold. PLoS One 2017;12(3):e0172327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteves JC, Marcantonio E Jr, de Souza Faloni AP, Rocha FR, Marcantonio RA, Wilk K, Intini G. Dynamics of bone healing after osteotomy with piezosurgery or conventional drilling - histomorphometrical, immunohistochemical, and molecular analysis. J Transl Med 2013;11:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabris ALDS, Faverani LP, Gomes-Ferreira PHS, Polo TOB, Santiago-Júnior JF, Okamoto R. Bone repair access of BoneCeramic™ in 5-mm defects: study on rat calvaria. J Appl Oral Sci 2018;26:e20160531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Lagunas J, Is the piezoelectric device the new standard for facial osteotomies? J Stomatol Oral Maxillofac Surg 2017; 118(4):255–8 [DOI] [PubMed] [Google Scholar]
- Gosain AK, Song L, Yu P, Mehrara BJ, Maeda CY, Gold LI & Longaker MT (2000) Osteogenesis in cranial defects: reassessment of the concept of critical size and the expression of TGF-beta isoforms. Plast Reconstr Surg 2000;106(2):360–71. [DOI] [PubMed] [Google Scholar]
- Gomes PS & Fernandes MH Rodent models in bone-related research: the relevance of calvarial defects in the assessment of bone regeneration strategies. Lab Anim 2011;45(1):14–24 [DOI] [PubMed] [Google Scholar]
- Hill CS, Luoma AM, Wilson SR, Kitchen N. Titanium cranioplasty and the prediction of complications. Brit J Neurosurg 2012;26(6):832–7. [DOI] [PubMed] [Google Scholar]
- Honeybul S. Neurological dysfunction due to large skull defect: implications for physioterapists. J Rehabil Med 2017;49(3):204–7. [DOI] [PubMed] [Google Scholar]
- Horton JE, Tarpley TM Jr, Jacoway JR. Clinical applications of ultrasonic instrumentation in the surgical removal of bone. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1981;51(3): 236–42. [DOI] [PubMed] [Google Scholar]
- Kerawala CJ, Martin IC, Allan W,Williams ED. The effects of operator technique and bur design on temperature during osseous preparation for osteosynthesis self-tapping screws. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;88(2): 145–50. [DOI] [PubMed] [Google Scholar]
- Landes CA, Stübinger S, Rieger J, Williger B, Ha TK, Sader R. Critical evaluation of piezoelectric osteotomy in orthognathic surgery: operative technique, blood loss, time requirement, nerve and vessel integrity. J Oral Maxillofac Surg 2008;66(4):657–74. [DOI] [PubMed] [Google Scholar]
- Pagotto LEC, de Santana Santos T, de Vasconcellos SJA, Santos JS, Martins-Filho PRS. Piezoelectric versus conventional techniques for orthognathic surgery: Systematic review and meta-analysis. J Craniomaxillofac Surg 2017;45(10):1607–13. [DOI] [PubMed] [Google Scholar]
- Piazza M, Grady MS. Cranioplasty. Neurosurg Clin N Am 2017;28(2):257–65. [DOI] [PubMed] [Google Scholar]
- Pryor ME, Polimeni G, Koo KT, Hartman MJ, Gross H, April M, Safadi FF, Wikesjö UM. Analysis of rat calvaria defects implanted with a platelet-rich plasma preparation: histologic and histometric observations. J Clin Periodontol 2005;32(9):966–72. [DOI] [PubMed] [Google Scholar]
- Robiony M, Polini F, Costa F, Vercellotti T, Politi M. Piezoelectric bone cutting in multipiece maxillary osteotomies. Technical note. J Oral Maxillofac Surg 2004;62(6): 759–61. [DOI] [PubMed] [Google Scholar]
- Russo A, Caravelli S, Mosca M, Girolami M, Ortolani A, Massimi S, Fuiano M, Zaffagnini S. Piezosurgery in hallux valgus correction: distal dinear osteotomy operative technique using piezoelectric tools. Joints 2019; 7(1):25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JP, Schwartz Z, Hollinger JO, Boyan BD. Characterization of rat calvarial nonunion defects. Acta Anat (Basel) 1990;138(3):185–92. [DOI] [PubMed] [Google Scholar]
- Spinelli G, Lazzeri D, Conti M, Agostini T, Mannelli G. Comparison of piezosurgery and traditional saw in bimaxillary orthognathic surgery. J Craniomaxillofac Surg 2014; 42(7):1211–20. [DOI] [PubMed] [Google Scholar]
- Stephan SJ, Tholpady SS, Gross B, Petrie-Aronin CE, Botchway EA, Nair LS, Ogle RC, Park SS. Injectable tissue-engineered bone repair of a rat calvarial defect. Laryngoscope 2010;120(5):895–901, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi K, Urist MR. The reaction of the dura to bone morphogenetic protein (BMP) in repair of skull defects. Ann Surg 1982;196(1):100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosun E, Bilgiç M, Yildirim B, Tüz HH, Özer T. Effects of Piezoelectric Surgery on Bone Regeneration Following Distraction Osteogenesis of Mandible. J Craniofac Surg 2017;28(1):74–8. [DOI] [PubMed] [Google Scholar]
- Vajgel A, Mardas N, Farias BC, Petri A, Cimões R, Donos N. A systematic review in a critical sized defect model. Clin Oral Implan Res 2014;25(8):879–93. [DOI] [PubMed] [Google Scholar]
- Williams L, Fan K, Bentley R. Titanium cranioplasty in children and adolescents. J Craniomaxillofac Surg 2016;44(7):789–94. [DOI] [PubMed] [Google Scholar]
- Wongsupa N, Nuntanaranont T, Kamolmattayakul S, Thuaksuban N. Assessment of bone regeneration of a tissue-engineered bone complex using human dental pulp stem cells/poly(ε-caprolactone)-biphasic calcium phosphate scaffold constructs in rabbit calvarial defects. J Mater Sci Mater Med 2017;28(5):77. [DOI] [PubMed] [Google Scholar]
- Youngstrom DW, Senos R, Zondervan RL, Brodeur JD, Lints AR, Young DR, Mitchell TL, Moore ME, Myers MH, Tseng WJ, Loomes KM, Hankenson KD. Intraoperative delivery of the Notch ligand Jagged-1 regenerates appendicular and craniofacial bone defects. NPJ Regen Med 2017;2:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Niu C, Fu X, Ding W, Ling S, Jiang X, Ji X. Early cranioplasty vs. late cranioplasty for the treatment of cranial defect: a systematic review. Clin Neurol Neurosurg 2015;136:33–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


