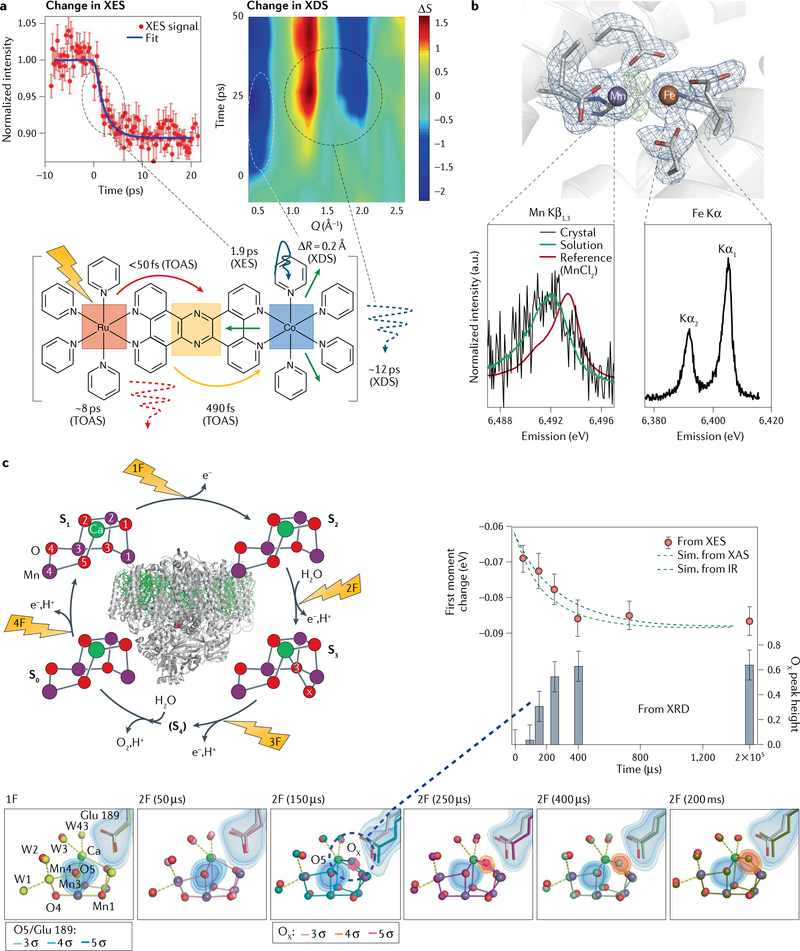
Fig. 3 |. Multimodal detection methods in X-ray free-electron laser studies using hard X-rays.
a | Measurements of femtosecond time-resolved Kα X-ray emission spectroscopy (XES) (left) in parallel with X-ray diffuse scattering (XDS) (right) on a Ru–Co dyad (bottom). The kinetics of the XES show the conversion of the low-spin to the high-spin form of Co within 2 ps. At early times, the XDS data exhibit a strong dip in intensity (ΔS) at momentum transfer Q = 0.5 Å−1, indicating an expansion by 0.2 Å on the 500-fs timescale; a second feature at higher Q indicates thermal equilibration at ~12 ps. The schematic also contains information derived from transient optical absorption spectroscopy (TOAS) measurements, indicating ultrafast electron transfer from Ru to the bridge and transfer from the bridge to Co in under 500 fs. b | X-ray diffraction (XRD) in combination with Mn Kβ and Fe Kα XES of ribonucleotide reductase. The structure of the dinuclear metal centre found in crystals of oxidized ribonucleotide reductase is shown. Electron density is contoured at 1.2 s in blue. Omit density (green) indicates the position of the bridging oxygen atoms. The Mn Kβ spectrum obtained from crystals and solutions is shown, together with a calibration spectrum of MnIICl2 (bottom left); the Fe Kα spectrum of the crystals is shown on the bottom right. c | Combined XRD and XES studies on photosystem II. Left: the overall structure of the protein and the four-step catalytic cycle (Kok cycle), revealed by flashes 1F–4F. For each of the stable states S0, S1, S2 and S3, the XRD structure of the catalytic Mn4Ca cluster obtained from X-ray free-electron laser measurements is also shown, exhibiting a change in the distance between Mn atoms 1 and 4 (given in Å). Right: results from time-resolved XES and XRD measurements, together with kinetic simulations based on previous infrared (IR) or X-ray absorption (XAS) measurements. Bottom: XRD and XES data show concomitant Mn oxidation and insertion of a new oxygen (OX) in the Mn cluster on the 250-μs timescale during the S2 → S3 transition. The S1 state structure is shown in light grey and the different time point structures in various colours (yellow to olive). The electron density is contoured at 3, 4 and 5 σ around the O5 and OX atoms of the Mn cluster and Glu189, a critical mobile amino acid side chain. Part a adapted with permission from REF.99. Part b adapted with permission from REF.18. Part c adapted with permission from REFS58,59.