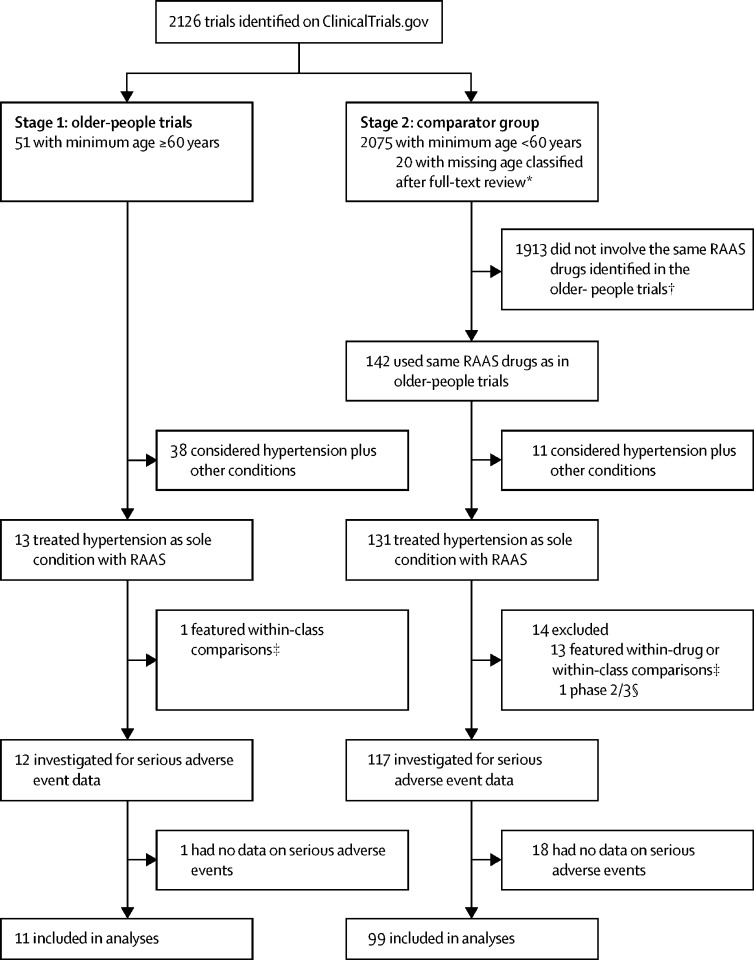
Figure 1.
Trial inclusion
ATC=Anatomic Therapeutic Chemical. RAAS=renin-angiotensin-aldosterone system. *The entry for the specific field in ClincialTrials.gov for the minimum age was missing. The full text of the trial registration was then reviewed to identify if the trial was targeted specifically at older participants. †All RAAS drugs were permitted for the selection of eligible older-people trials. Only drugs that were studied in one or more of the older-people trials (aliskiren, irbesartan, olmesartan, telmisartan, or valsartan) were selected for the comparator group of the standard trials. ‡Within-drug comparisons refers to trials where all arms included the same drug (eg, trials of different dosages or regimens). Within-class comparisons refers to trials where all arms included drugs with the same five-character ATC class (eg, drugs in WHO ATC class C09CA are all angiotensin II receptor blockers). §Excluded because none of the older-people trials were phase 2/3.