Abstract
Milk fat globule membrane (MFGM) possesses various nutritional and biological benefits for mammals, whereas its effects on neonatal gut microbiota and barrier integrity remained unclear. This study investigated the effects of MFGM administration on microbial compositions and intestinal barrier functions of neonatal piglets. Sixteen newborn piglets were randomly allocated into a CON group or MFGM group, orally administered with saline or MFGM solution (1 g/kg body weight) respectively during the first postnatal week, and all piglets were breastfed during the whole neonatal period. The present study found that the MFGM oral administration during the first postnatal week increased the plasma immunoglobulin (Ig) G level, body weight and average daily gain of piglets (P < 0.05) on 21 d. Additionally, MFGM administration enriched fecal SCFA-producing bacteria (Ruminococaceae_UCG-002, Ruminococaceae_UCG-010, Ruminococaceae_UCG-004, Ruminococaceae_UCG-014 and [Ruminococcus]_gauvrearuii_group), SCFA concentrations (acetate, propionate and butyrate; P < 0.05) and their receptor (G-protein coupled receptor 41, GPR41). Furthermore, MFGM administration promoted intestinal villus morphology (P < 0.05) and barrier functions by upregulating genes of tight junctions (E-cadherin, claudin-1, occludin and zonula occludin 1 [ZO-1]), mucins (mucin-13 and mucin-20) and interleukin (IL)-22 (P < 0.05). Positive correlation was found between the beneficial microbes and SCFA levels pairwise with the intestinal barrier genes (P < 0.05). In conclusion, orally administrating MFGM during the first postnatal week stimulated SCFA-producing bacteria colonization and SCFA generation, enhanced intestinal barrier functions and consequently improved growth performance of neonatal piglets on 21 d. Our findings will provide new insights about MFGM intervention for microbial colonization and intestinal development of neonates during their early life.
Keywords: Milk fat globule membrane, Gut microbiota, SCFA, Intestinal barrier, Piglet
1. Introduction
After birth, the gastrointestinal tract of mammal neonates are required to structurally and functionally adapt to the extrauterine environment and oral diets, cultivating the garden for colonizing bacteria (Buddington and Sangild, 2011). Growing evidence indicates that neonatal bacteria colonization as well as the interactions between the gut microbes and the host can have profound effects on host health outcomes (Nylund,et al., 2014; Tamburini et al., 2016; Deguine, 2018). Intestinal microbiota have vital functions on gut barriers, metabolic reactions, nutritional effects and the maturation of the host's immune responses (Rowland et al., 2017; Ma et al., 2018; Valdes et al., 2018). Thus, optimal intestinal development coupled with microbial colonization during the neonatal period is of crucial importance for health and growth in mammals.
Microbial colonization plays an indispensable role in barrier functions and immunological maturation as a substantial contributor to intestinal development (Inoue et al., 2005; Lin and Zhang, 2017). On the one hand, luminal microbes and their metabolites, such as short chain fatty acids (SCFA), can directly facilitate intercellular tight junctions, building up the primary barrier (Kelly et al., 2015; Singh et al., 2019). On the other hand, microorganisms can also alter epithelial permeability by stimulating the host to release cytokines, mucins and antimicrobial peptides, indirectly eliminating pathogens and enhancing barrier functions (McGuckin,et al., 2011; McDermott and Huffnagle, 2014; Ageitos et al., 2017). Therefore, exploring new nutritional strategies that can program the microbial population appropriately is crucial to further improve the intestinal development of neonates.
Milk fat globule membrane (MFGM) is a highly structured complex, containing unique polar lipids and membrane-specific proteins, enveloping fat globules secreted from epithelial cells of the mammary gland (Bourlieu and Michalski, 2015), which can increase the nutritional bioavailability for intestinal growth and development (Demmelmair,et al., 2017). MFGM supplementation showed antimicrobial advantages in shifting the microbial community by selectively inhibiting the adherence of pathogens (Atroshi et al., 1983; Guerin et al., 2018). MFGM improved gut development by protecting intestinal integrity from infections in the lipopolysaccharide (LPS)-induced model (Park et al., 2010; Snow et al., 2011). Furthermore, MFGM was able to balance the host immune status through its immunomodulatory effects on secretion of cytokines and immunoglobulins (Zanabria,et al., 2014; Huang et al., 2019). Moreover, MFGM fractions might serve as one of the key regulators in metabolic activities, especially in lipid-associated metabolism (Huerou-Luron,et al., 2018; Teller et al., 2018). MFGM intervention could improve the neurocognitive functions of neonates, thereby achieving a similar performance as breastfed infants (Timby,et al., 2014; Brink and Lonnerdal, 2018; Brink et al., 2019). Besides, our previous study confirmed the maternal imprinting effect of MFGM on microbiota and the intestinal barrier functions of piglets (Zhang et al., 2020). However, the impacts of administrating MFGM directly to neonatal piglets on their intestinal health and growth performances were still unclear.
Considering the critical significance of microbial colonization in the neonatal period and the multidimensional benefits of MFGM for intestinal health and development, this study was conducted to investigate the effects and potential mechanisms of early life MFGM administration on gut microbial composition, intestinal barrier functions and growth performance of neonatal piglets.
2. Materials and methods
2.1. Animals and experimental design
The animal use protocols were reviewed and approved by the China Agricultural University Animal Care and Use Committee (AW07040202-1, Beijing, China). All procedures were performed strictly according to the Guide for Experimental Animals of the Ministry of Science and Technology (Beijing, China).
A total of 16 newborn piglets (Duroc × Landrace × Yokshire, 1.53 ± 0.04 kg) from different litters (one piglet per litter) were assigned into a CON group or MFGM group. Piglets in the MFGM group were administered with 5 mL MFGM solution (1 g/kg body weight, provided by Beijing Sanyuan Foods Co. Ltd, Beijing, China) per day during 1 d to 7 d of the postnatal period. At the same time, piglets in the CON group were administered with equal physiological saline. The MFGM solution and saline were infused into the piglet mouth by an injector with medical sterile tube. The piglets were allowed ad libitum access to sow milk and water throughout the neonatal period. Commercial creep feed was added from 8 d postpartum. Health status was monitored daily and body weight was recorded weekly until 21 d.
2.2. Sample collection
On 21 d of the neonatal period, 5 piglets per group (approximately to the average body weight of each group) were randomly selected, and their blood was sampled from the jugular vein. Plasma was carefully collected after centrifuging at 3,000 × g at 4 °C for 10 min. Then, feces were collected and snap-frozen in liquid nitrogen for microbiota composition analysis. As piglets were humanely euthanized, duodenum, jejunum and ileum samples were fixed in 10% phosphate-buffered formalin for morphological evaluation. The colonic content was gently scraped down, collected into sterile tubes and snap-frozen in liquid nitrogen for SCFA analysis. For mucosal samples, after rinsing the intestinal content, mid-ileal and mid-colonic mucosa were scraped gently by glass slide, collected into the sterile RNAase-free tubes and frozen in liquid nitrogen for gene expressions identification. All samples were stored at −80 °C until further analysis.
2.3. Plasma parameters measurement
All plasma samples were thawed and completely mixed before analysis. The biochemical parameters were measured by an automatic biochemical analyzer (Hitachi 7160, Hitachi High-Technologies Corporation, Tokyo, Japan). To investigate the intestinal permeability and immune status, the plasma diamine oxidase (DAO) level and plasma immunoglobulins (including IgA, IgG and IgM) concentrations were respectively measured by ELISA according to the manufacturer's instructions (Beijing Sino-UK Institute of Biological Technology, Beijing, China).
2.4. Intestinal morphology detection
Intestinal samples were removed from 10% phosphate-buffered formalin and dehydrated through a graded ethanol series (70% to 100%), then cleared with xylene and embedded in paraffin wax. Serial sections (5 μm thickness) were cut by LEICA RM2135 rotary microtome (Leica Microsystems GmbH, Simi Valley, California, United States), and stained with hematoxylin and eosin. A minimum of 15 intact and well-oriented villi and their associated crypts from each segment were measured at 100× magnifications under bright field on a Zeiss Axio Imager microscope (Carl Zeiss Microscopy LLC, White Plains, New York, United States). Villus height was measured from the tip of the villi to the villus crypt junction, and crypt depth was defined as the depth of the invagination between adjacent villi (Huang et al., 2019).
2.5. Microbial sequencing analysis
Total genomic DNA of fecal samples was extracted using the QIAamp Fast DNA Stool Mini Kit (Qiagen, Tübingen, Germany). The V3 to V4 regions of the 16S rRNA gene were amplified using universal primers (Ren et al., 2018) and purified by AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, California, United States). Then, the purified PCR products were pooled into equimolar amounts and sequenced on the Illumina HiSeq 2500 platform to generate paired end reads of 300 bp.
Raw paired-end reads were strictly analyzed using QIIME (version 1.9). In brief, the low-quality sequences were removed and the remaining high-quality sequences were clustered into operational taxonomic unit (OTU) with 97% similarity. The taxonomy assignment of OTU was conducted with the RDP classifier against the SILVA 16S rRNA gene database (Release 132) with a confidence threshold value of 0.70. The number of OTU per sample was calculated as the proxy for alpha-diversity by using the MOTHUR program. For beta-diversity analysis, principal coordinates analysis (PCoA) was performed based on Unweighted Unifrac distances using QIIME, and ANOSIM (1,000 Monte Carlo permutations) was used for the statistical analysis. Wilcoxon rank-sum-test was applied to find out the different bacteria between two groups. For microbial functional prediction, PICRUSt was performed to analyze the differential abundant KEGG pathways. The data were analyzed on the free online platform of Majorbio Cloud Platform (www.majorbio.com).
2.6. SCFA concentrations measurement
Short chain fatty acids including acetate, propionate, and butyrate in colonic content were quantified using Ion Chromatograph as previously described (He et al., 2018). In brief, 0.5-g colonic content samples were weighed and dissolved with 8 mL ultrapure water to homogenize, and then centrifuged at 5,000 × g for 10 min. After this, the supernatants were diluted as 1:50 and filtered through a 0.22-μm membrane, and then subjected to Ion Chromatography System (DIONEX ICS-3000, Thermo Fisher Scientific, Waltham, Massachusetts, United States) for SCFA measurement.
2.7. Gene expressions quantification
Total RNA of mid-ileum mucosa was extracted by Trizol reagent (Invitrogen, United States) according to the protocol. cDNA was obtained by reverse transcription PCR (RT-PCR) on a T100 Thermal Cycler according to PrimeScript RT reagent Kit (Takara, Shiga, Japan). Quantitative reverse transcription PCR (RT-qPCR) was performed according to the SYBR Premix Ex Taq II instructions (Takara, Shiga, Japan) on a Light Cycler System (Roche, South San Francisco, California, United States). The reaction systems and programs of RT-PCR and RT-qPCR are shown in the Appendix. Primers for RT-qPCR (Appendix Table 1) were synthesized by Generay Company (Shanghai, China). Amplifications were performed in triplicate for each sample. The relative expression of target genes to that of the reference gene (GAPDH) was calculated according to the 2−ΔΔCt method.
2.8. Statistical analysis
Data were analyzed by SPSS 20.0 (IBM, United States) and the results are shown as mean ± SEM (standard error of mean). Student's t test was used for determining the statistical differences. GraphPad Prism (version 7, GraphPad Software, United States) was used for the graphical representations; all statistical analyses were considered significant at P < 0.05.
3. Results
3.1. Effects of MFGM administration on growth performance of the neonatal piglets
As shown in Table 1, compared to the piglets in the CON group, piglets with MFGM administration in the first week had significantly higher body weight on 14 and 21 d (P < 0.05). Further, the average daily gains of piglets between 8 and 14 d, 15 to 21 d and throughout the whole neonatal period (1 to 21 d) were elevated in the MFGM group (P < 0.05).
Table 1.
Effects of milk fat globule membrane (MFGM) administration on growth performance of the neonatal piglets.
| Item | Group1 |
P-value | |
|---|---|---|---|
| CON | MFGM | ||
| Body weight, kg | |||
| 7 d | 2.47 ± 0.08 | 2.67 ± 0.14 | 0.203 |
| 14 d | 4.18 ± 0.09 | 4.74 ± 0.17 | 0.010 |
| 21 d | 5.70 ± 0.15 | 6.77 ± 0.27 | 0.003 |
| Average daily gain, g | |||
| 1 to 7 d | 134.37 ± 9.64 | 162.76 ± 20.78 | 0.218 |
| 8 to 14 d | 244.11 ± 14.61 | 294.90 ± 17.97 | 0.045 |
| 15 to 21 d | 217.32 ± 19.08 | 289.80 ± 28.53 | 0.050 |
| 1 to 21 d | 198.60 ± 7.22 | 249.15 ± 12.98 | 0.004 |
CON, piglets fed with saline; MFGM, piglets fed with MFGM.
3.2. Effects of MFGM administration on plasma parameters of the neonatal piglets on 21 d
The concentration of plasma IgG on 21 d was significantly increased, whereas the plasma DAO level was decreased in piglets with MFGM administration in the first week (P < 0.05). No significant differences were found in other parameters including glucose (GLU), IgA, IgM, triglyceride (TG), high density lipoprotein-cholesterol (HDL-C) and low density lipoprotein-cholesterol (LDL-C) between the two groups (Table 2).
Table 2.
Effects of milk fat globule membrane (MFGM) administration on plasma parameters of the neonatal piglets on 21 d.
| Item | Group1 |
P-value | |
|---|---|---|---|
| CON | MFGM | ||
| GLU, mmol/L | 7.33 ± 0.46 | 6.36 ± 0.07 | 0.128 |
| IgA, g/L | 0.85 ± 0.06 | 0.92 ± 0.05 | 0.379 |
| IgG, g/L | 7.08 ± 0.34 | 9.20 ± 0.30 | 0.001 |
| IgM, g/L | 1.02 ± 0.06 | 0.90 ± 0.09 | 0.257 |
| TG, mmol/L | 1.18 ± 0.08 | 1.11 ± 0.11 | 0.658 |
| HDL-C, mmol/L | 1.37 ± 0.12 | 1.39 ± 0.08 | 0.910 |
| LDL-C, mmol/L | 2.08 ± 0.09 | 2.20 ± 0.21 | 0.650 |
| DAO, U/mL | 3.57 ± 0.31 | 2.62 ± 0.07 | 0.015 |
GLU = glucose; IgA = immunoglobulin A; IgG = immunoglobulin G; IgM = immunoglobulin M; TG = triglyceride; HDL-C = high density lipoprotein-cholesterol; LDL-C = low density lipoprotein-cholesterol; DAO = diamine oxidase.
CON, piglets fed with saline; MFGM, piglets fed with MFGM.
3.3. Effects of MFGM administration on intestinal morphology of the neonatal piglets on 21 d
To define the intestinal morphological development of piglets, villus height and crypt depth of intestines were measured (Fig. 1 and Table 3). Results showed that administrating MFGM in the first week increased the villus height of the duodenum and ileum, but decreased the crypt depth of the duodenum of piglets on 21 d (P < 0.05). In addition, the ratio of intestinal villus height to crypt depth was significantly higher in the MFGM group than in the CON group (P < 0.05).
Fig. 1.
Effects of milk fat globule membrane (MFGM) administration on intestinal morphological structure of the neonatal piglets on 21 d. Light microscopy (100×) of the intestinal morphology of duodenum, jejunum and ileum in piglets. CON, piglets fed with saline; MFGM, piglets fed with MFGM.
Table 3.
Effects of milk fat globule membrane (MFGM) administration on intestinal morphology of the neonatal piglets on 21 d.
| Item | Group1 |
P-value | |
|---|---|---|---|
| CON | MFGM | ||
| Villus height, μm | |||
| Duodenum | 416.70 ± 18.09 | 480.45 ± 19.20 | 0.042 |
| Jejunum | 423.50 ± 22.58 | 399.00 ± 18.86 | 0.429 |
| Ileum | 342.78 ± 33.80 | 444.68 ± 19.04 | 0.030 |
| Crypt depth, μm | |||
| Duodenum | 184.92 ± 13.69 | 112.93 ± 5.35 | 0.001 |
| Jejunum | 135.43 ± 10.27 | 107.07 ± 9.71 | 0.080 |
| Ileum | 102.95 ± 5.78 | 111.33 ± 9.30 | 0.466 |
| Villus height-to-crypt depth ratio | |||
| Duodenum | 2.32 ± 0.24 | 4.63 ± 0.52 | 0.004 |
| Jejunum | 3.21 ± 0.31 | 3.62 ± 0.25 | 0.336 |
| Ileum | 3.71 ± 0.52 | 4.47 ± 0.52 | 0.332 |
CON, piglets fed with saline; MFGM, piglets fed with MFGM.
3.4. Effects of MFGM administration on fecal microbial composition and functional profiles of the neonatal piglets on 21 d
The differences in bacteria community compositions of piglets in the CON and MFGM groups were investigated. A total of 844,360 high-quality sequences were obtained from 10 samples. Based on 97% sequence similarity, 907 OTU were identified and then assigned to 20 phyla, 28 classes, 54 orders, 106 families, and 291 genera. In spite of the 714 common OTU, 75 OTU were solely isolated in the CON group and 118 OTU were present in the MFGM group (Fig. 2A).
Fig. 2.
Effects of milk fat globule membrane administration on fecal microbiota composition of the neonatal piglets on 21 d. Venn diagram (A); beta-diversity of PCoA based on Unweighted Unifrac distances (B); microbial composition at the phylum and genus levels (C, D); differential microbial composition based on Wilconxon rank sum test (E). CON, piglets fed with saline; MFGM, piglets fed with milk fat globule membrane. ∗P < 0.05.
In terms of the alpha-diversity (Table 4), Simpson indexes were significantly decreased in the MFGM group (P < 0.05), whereas no differences in microbial Sobs index, Ace index, Chao index and Shannon index were observed between the two groups. For the beta-diversity, principal coordinates analysis (PCoA) based on the Unweighted Unifrac distances showed significant differences between the CON group and the MFGM group (Fig. 2B).
Table 4.
Effects of milk fat globule membrane (MFGM) administration on fecal bacteria alpha-diversity of the neonatal piglets on 21 d.
| Item | Group1 |
P-value | |
|---|---|---|---|
| CON | MFGM | ||
| Sobs index | 526.60 ± 52.25 | 583.60 ± 48.15 | 0.112 |
| Ace index | 618.08 ± 52.62 | 667.85 ± 48.41 | 0.158 |
| Chao index | 637.85 ± 51.79 | 679.66 ± 65.66 | 0.300 |
| Shannon index | 3.59 ± 0.58 | 4.25 ± 0.41 | 0.072 |
| Simpson index | 0.10 ± 0.05 | 0.04 ± 0.02 | 0.037 |
CON, piglets fed with saline; MFGM, piglets fed with MFGM.
The bar plots of microbiota composition showed that Firmicutes and Bacteroidetes were the predominated phyla in piglets of both the CON and MFGM group (Fig. 2C). At genus level, Bacteroides was the most dominated microbiota in the CON group, whereas Ruminococaceae_UCG-002 was the most dominated microbiota in the MFGM group (Fig. 2D).
Differential microbiota analyzed by Wilconxon rank-sum test at genus level demonstrated that administrating MFGM in the first week significantly increased the relative abundances of Ruminococaceae_UCG-002, Ruminococaceae_UCG-010, Ruminococaceae_UCG-004, Ruminococaceae_UCG-014 and [Ruminococcus]_gauvrearuii_group, but decreased Tyzzerella, Clostridium_sensu_stricto_2 and Eisenbergiella on 21 d (P < 0.05) (Fig. 2E).
To investigate the functional profiles of the bacterial community, Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) was applied to estimate the gene family abundances of bacterial communities (Fig. 3). The results showed that the relative abundances of genes involved in the secretion system, sporulation, phenylalanine, tyrosine and tryptophan biosynthesis, proteins replication, recombination and repair proteins, pantothenate and CoA biosynthesis, propionate metabolism and signal transduction mechanisms were significantly elevated in the piglets from the MFGM group (P < 0.05), whereas those involved in glycine, serine and threonine metabolism, carbon fixation in photosynthetic organisms, glyoxylate and dicarboxylate metabolism and LPS biosynthesis proteins were significantly declined in the piglets from the MFGM group (P < 0.05).
Fig. 3.
Effects of milk fat globule membrane (MFGM) administration on functional prediction of microbial community of the neonatal piglets on 21 d. Differential abundances of KEGG pathways. KEGG = Kyoto Encyclopedia of Genes and Genomes. CON, piglets fed with saline; MFGM, piglets fed with MFGM. ∗P < 0.05; ∗∗P < 0.01.
3.5. Effects of MFGM administration on fecal SCFA concentrations and intestinal GPR gene expressions of the neonatal piglets on 21 d
The SCFA concentrations in the feces and their receptor gene expressions in the intestine were tested. The results showed that the concentrations of acetate, propionate and butyrate in the MFGM group were higher than those in the CON group (P < 0.05) (Fig. 4A). Additionally, administering MFGM in the first week increased the gene expression of G-protein coupled receptor 41 (GPR41) in the colonic mucosa on 21 d compared to the CON group (P < 0.05) (Fig. 4B).
Fig. 4.
Effects of milk fat globule membrane administration on fecal SCFA concentrations and intestinal GPR genes of the neonatal piglets on 21 d. The concentrations of SCFA in colonic content (A); the gene expressions of GPRs in colonic mucosa (B). CON, piglets fed with saline; MFGM, piglets fed with milk fat globule membrane. GPR41 = G-protein coupled receptor 41; GPR43 = G-protein coupled receptor 43; GPR119 = G-protein coupled receptor 119; GPR120 = G-protein coupled receptor 120. ∗P < 0.05.
3.6. Effects of MFGM administration on intestinal barrier functions associated genes of the neonatal piglets on 21 d
The gut barrier functions in the ileum and colon of piglets were evaluated to define the intestinal development. The results clarified that administration of MFGM in the first week significantly upregulated the gene expressions of tight junctions (E-cadherin, occludin, claudin-4 and zonula occludin 1 [ZO-1]), mucins (mucin-13 and mucin-20) and interleukin (IL)-22 in the ileum (P < 0.05) (Fig. 5A–C); gene expressions of claudin-1, IL-22 and regenerating gene 3γ (Reg3γ) were significantly elevated in the MFGM group (P < 0.05) (Fig. 5D–F).
Fig. 5.
Effects of milk fat globule membrane administration on intestinal barrier functions associated genes of the neonatal piglets on 21 d. Gene expressions of tight junctions, mucins, cytokines and Reg3γ in ileal mucosa (A to C) and colonic mucosa (D to F). CON, piglets fed with saline; MFGM, piglets fed with milk fat globule membrane. ZO-1 = zonula occludin 1; TNF-α = tumor necrosis factor α; IFN-γ = interferon γ; IL-1β = interleukin 1β; IL-10 = interleukin 10; IL-22 = interleukin 22; Reg3γ = regenerating gene 3γ. ∗P < 0.05.
3.7. Spearman correlation analysis between microbiota and intestinal barrier functions associated genes, SCFA concentrations
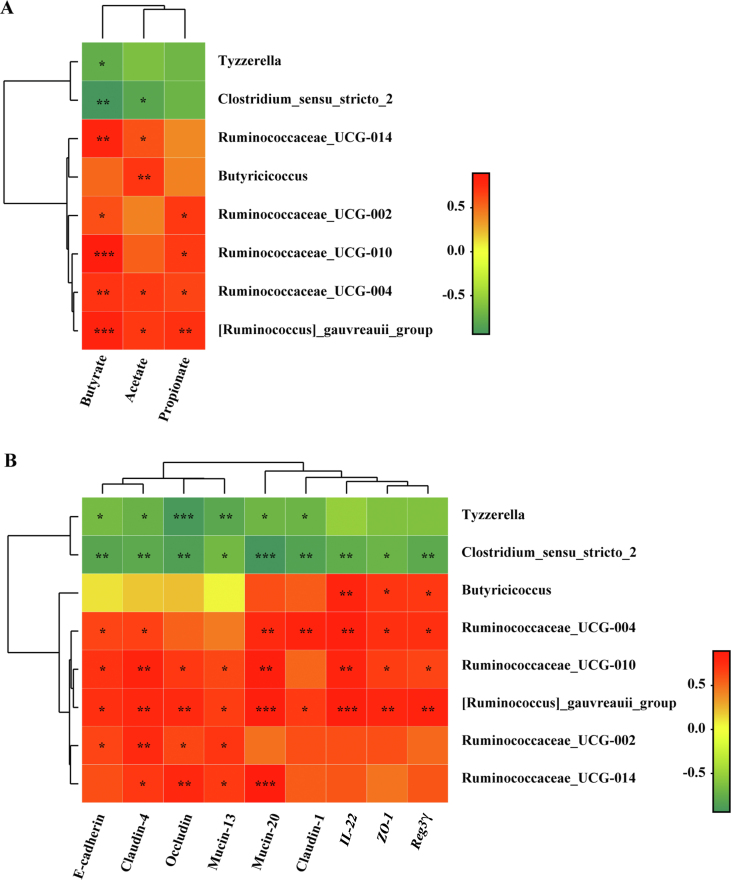
To further affirm microbial effects on gut barrier functions and SCFA production, spearman correlation analyses were carried out. The results exhibited that the enriched bacteria (mainly located in Ruminococcaceae) in the MFGM group was positively correlated with SCFA concentrations (P < 0.05) (Fig. 6A) and gene expressions of barrier functions (P < 0.05) (Fig. 6B) separately, whereas decreased bacteria in the MFGM group showed strong negative correlation with the barrier function genes and SCFA levels (P < 0.05).
Fig. 6.
Spearman correlation analysis between microbiota and SCFA concentrations, intestinal barrier functions associated gene expressions. The correlation heatmap of microbiota and SCFA levels in feces (A), as well as intestinal barrier genes (B). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
4. Discussion
After birth, mammal intestinal morphology and functionality are obliged to adapt into a bacteria-rich environment from a germ-free uterus (Guilloteau et al., 2010). Merging evidence has highlighted that the neonatal period is a critical window phase for microbial colonization which can have profound impacts on the intestinal health and development throughout an animals’ lifetime (Tamburini et al., 2016; Deguine, 2018). MFGM, a lipid–protein complex enveloping milk fat globule, was reported to exert profitable effects on digestion, physiology and modulation in gut microbial populations (Sprong et al., 2012; Hernell et al., 2019). In the present study, oral administration of MFGM during the first week improved SCFA production by facilitating the SCFA-producing bacteria colonization, enhancing intestinal barrier functions and consequently improving the growth performance of neonatal piglets.
MFGM, mainly structured with glycoproteins, phospholipids and sphingolipids, was regarded as a potential nutraceutical possessing healthy biological benefits to the mammals (Dewettinck et al., 2008, Spitsberg, 2005). Butyrophilin (BTN), mucins, xanthine oxidoreductase (XOR), lactadherin (MFG-E8) and fatty acid binding protein (FABP) are well characterized MFGM proteins with various biochemical properties (Demmelmair,et al., 2017). Previous study has demonstrated that dietary MFGM supplementation exerted growth-enhancing effects on neonatal health and intestinal maturation of infants and young animals, which confirmed our results of the promoted growth performance of piglets after the first week of MFGM administration (Bhinder,et al., 2017). Moreover, the elevated plasma IgG level in the present study indicated that administrating MFGM in the first week strengthened the immunological defense of neonatal piglets and prevented infections (Iwasaki and Medzhitov, 2015).
Numerous studies have demonstrated that MFGM fractions contributed to the alteration in gut microbial composition (Bhinder,et al., 2017; He et al., 2019). On the one hand, some glycosylated proteins from MFGM could provide substrates for microbial fermentation, and the metabolites in return may affect the microbiota (Bourlieu and Michalski, 2015). In the present study, administration of MFGM in the first week enriched the beneficial SCFA-producing Ruminococcaceae and Ruminococcus on 21 d, which could ferment milk oligosaccharides to produce SCFA for microbial modulation and epithelial development (Paturi,et al., 2018). On the other hand, glycosylated proteins and sphingolipids from MFGM acted as binding sites or stimulate mucin secretion, thereby preventing the adherence of pathogens to intestinal mucosa (Huerou-Luron,et al., 2018). In the current study, the declination of Tyzzerella, Clostridium_sensu_stricto_2 and Eisenbergiella, along with the upregulation of mucins (mucin-13 and mucin-20) in MFGM group implied that MFGM helped to build up the mucus layer to suppress the colonization of pathogens. Additionally, as a biomarker of pathogens, the decreased bacteria functional profiles involved in LPS biosynthesis proteins in the MFGM group also inhibited the pathogenic proliferation and colonization (Battle et al., 2015), and subsequently improved intestinal health and barrier functions.
Furthermore, some of the specific MFGM fractions could interact with the nutrients and microbial metabolism, maintaining metabolic homeostasis (Conway et al., 2014). For example, FABP, a well described protein present in high concentration in MFGM, plays an active role in fatty acids bonding, as fatty acids enter the cells for further fatty acid metabolism (Hotamisligil and Bernlohr, 2015) . In the present study, the increases in the SCFA concentrations and their receptor (GPR41) in the MFGM group, as well as the promoted propionate metabolism profiles, indicated that administrating MFGM in the first week regulated the microbiota and SCFA metabolism, which eventually energized the enterocytes and enhanced the gut barrier functions (Kelly et al., 2015). In addition, the modification in biosynthesis and metabolism of some amino acids, such as phenylalanine, tyrosine and tryptophan in the MFGM group were also reported to contribute to intestinal integrity and health (Mou,et al., 2019). Besides, the increased pantothenate and CoA biosynthesis profiles may accelerate energy production, contributing to the intestinal homeostasis and growth development (Grevengoed et al., 2014).
MFGM fractions have been shown to have various advantageous impacts on intestinal integrity and barrier functions. The intestinal barrier is composed of a physical barrier, chemical barrier, immunological barrier and a microbial barrier. Tight junctions and mucins build up the primary barrier for preventing the invasion of pathogens (Pelaseyed et al., 2014; Halpern and Denning, 2015). In the current study, the upregulated gene expressions of tight junctions (E-cadherin, occludin, claudin-4 and ZO-1) and mucins (mucin-13 and mucin-20) in the MFGM group suggested that the intestinal physical and chemical barriers that protect intestines from pathogenic infection were enhanced, which is consistent with the results of previous studies (Park et al., 2010; Li et al., 2018a, b). In addition, the modified immunological barrier due to the increase of IL-22, contributed to the activation of the mucosa immune defense, thereby improving resistance to colonization of pathogens and intestinal health (Schreiber et al., 2015; Sovran et al., 2015). Additionally, IL-22 augmentation was shown to induce the antimicrobial peptide Reg3γ expression, which is an important component of the chemical barrier involved in the retardation of pathogens (Sonnenberg et al., 2011). The colonization of SCFA-producing bacteria in the MFGM group (mainly classified in Ruminococcaceae) was supportive to the gut microbial barrier. A positive relationship between the settled bacteria and the intestinal barrier genes demonstrated the indispensable role of SCFA-producing bacteria in the intestinal barrier establishment (Perdijk et al., 2019). Meanwhile, the bacteria-derived SCFA were reported to not only directly enhance the intestinal barriers (Elamin,et al., 2013), but also confer healthy outcomes by upregulating Reg3γ expressions (Chun et al., 2019; Bajic et al., 2020). Furthermore, lower plasma DAO levels of piglets in the MFGM group confirmed the superior intestinal barrier functions with inferior permeability after the first week of MFGM administration, because DAO as an intracellular component would be released into the blood as the intestinal permeability increased (Qin et al., 2019).
Previous studies have confirmed the barrier-enhancing role of MFGM intervention in different animal models (Li et al., 2018a, b; Huang et al., 2019). On the one hand, the MFGM fractions, such as gangliosides and MFG-E8, might directly promote the intestinal barriers (Park et al., 2010; Li et al., 2018a, b). On the other hand, MFGM modulated the commensals colonization and adhesion of pathogens, the resident bacteria and their metabolites, which could then contribute to the intestinal barrier functions (Guerin et al., 2018; Bhinder et al., 2017). In the present study, it could be inferred that MFGM intervention during only the first week might shape the microbial colonization in the early life, which showed a long-term effect on the microbial populations and their metabolic status on 21 d. The colonized SCFA-producing bacteria and the produced SCFA have been reported to improve the gut barrier functions (Elamin,et al., 2013).
5. Conclusions
Collectively, oral administration of MFGM during the first postnatal week not only enhanced the piglets’ growth over the duration, but also exhibited a long-term effect on the immunological defense, microbial colonization, intestinal barriers and growth performance until 21 d. Early life administration of MFGM elevated the plasma IgG level, activated SCFA-producing bacteria colonization (i.e. Ruminococcaceae) for generating more SCFA, and promoted intestinal morphology and barrier functions by upregulating gene expressions of tight junctions (E-cadherin, claudin-1, occludin and ZO-1), mucins (mucin-13 and mucin-20) and anti-inflammatory cytokines (IL-22) on 21 d. Our findings suggest MFGM as a potential functional ingredient for modulating microbial populations and improving intestinal health and functions in mammalian neonates.
Author contributions
Yujun Wu: Data curation, Investigation, Validation, Visualization, Writing – original draft; Xiangyu Zhang: Data curation, Investigation, Validation; Dandan Han: Supervision, Writing – review & editing; Shiyu Tao: Visualization, Writing – review & editing; Yu Pi: Visualization, Writing – review & editing; Shiyi Zhang: Writing – review & editing; Shilan Wang: Methodology, Formal analysis; Junying Zhao: Software, Resources; Lijun Chen: Conceptualization, Resources, Supervision, Visualization,; Junjun Wang: Conceptualization, Funding acquisition, Project administration, Supervision, Visualization, Writing – review & editing.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that might inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
We appreciated Beijing Sanyuan Foods Co. Ltd. (Beijing, China) for donation of the MFGM. This research was financially supported by the Beijing Municipal Natural Science Foundation (S170001), the National Natural Science Foundation of China (31630074, 31902170, 31972596 and 31902189), the National Key Research and Development Program of China (2016YFD0500506 and 2018YDF0501002), the Agriculture Research System of China (CARS-35), the Higher Education Discipline Innovation Project (B16044).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aninu.2020.07.012.
Contributor Information
Lijun Chen, Email: chenlijun@sanyuan.com.cn.
Junjun Wang, Email: wangjj@cau.edu.cn.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- Ageitos J.M., Sanchez-Perez A., Calo-Mata P., Villa T.G. Antimicrobial peptides (AMPs): Ancient compounds that represent novel weapons in the fight against bacteria. Biochem Pharmacol. 2017;133:117–118. doi: 10.1016/j.bcp.2016.09.018. [DOI] [PubMed] [Google Scholar]
- Atroshi F., Alaviuhkola T., Schildt R., Sandholm M. Fat globule-membrane of sow milk as a target for adhesion of K88-positive Escherichia-coli. Comp Immunol Microb. 1983;6(3):235–245. doi: 10.1016/0147-9571(83)90016-4. [DOI] [PubMed] [Google Scholar]
- Bajic D., Niemann A., Hillmer A.K., Mejias-Luque R., Bluemel S., Docampo M. Gut microbiota derived propionate regulates the expression of Reg 3 mucosal lectins and ameliorates experimental colitis in mice. J Crohns Colitis. 2020;14(10):1462–1472. doi: 10.1093/ecco-jcc/jjaa065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battle A., Khan Z., Wang S.H., Mitrano A., Ford M.J., Pritchard J.K. Genomic variation. Impact of regulatory variation from RNA to protein. Science. 2015;347(6222):664–667. doi: 10.1126/science.1260793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhinder G., Allaire M., Garcia C., Lau J.T., Chan J.M., Ryz N.R. Milk fat globule membrane supplementation in formula modulates the neonatal gut microbiome and normalizes intestinal development. Sci Rep. 2017;7:45274. doi: 10.1038/srep45274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourlieu C., Michalski M.C. Structure-function relationship of the milk fat globule. Curr Opin Clin Nutr Metab Care. 2015;18(2):118–127. doi: 10.1097/MCO.0000000000000138. [DOI] [PubMed] [Google Scholar]
- Brink L.R., Gueniot J.P., Lonnerdal B. Effects of milk fat globule membrane and its various components on neurologic development in a postnatal growth restriction rat model. J Nutr Biochem. 2019;69:163–171. doi: 10.1016/j.jnutbio.2019.03.013. [DOI] [PubMed] [Google Scholar]
- Brink L.R., Lonnerdal B. The role of milk fat globule membranes in behavior and cognitive function using a suckling rat pup supplementation model. J Nutr Biochem. 2018;58:131–137. doi: 10.1016/j.jnutbio.2018.05.004. [DOI] [PubMed] [Google Scholar]
- Buddington R.K., Sangild P.T. Companion animals symposium: Development of the mammalian gastrointestinal tract, the resident microbiota, and the role of diet in early life. J Anim Sci. 2011;89(5):1506–1509. doi: 10.2527/jas.2010-3705. [DOI] [PubMed] [Google Scholar]
- Chun E., Lavoie S., Fonseca-Pereira D., Bae S., Michaud M., Hoveyda H.R. Metabolite-sensing receptor ffar2 regulates colonic group 3 innate lymphoid cells and gut immunity. Immunity. 2019;51(5):871–874. doi: 10.1016/j.immuni.2019.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway V., Gauthier S.F., Pouliot Y. Buttermilk: Much more than a source of milk phospholipids. Anim Front. 2014;4(2):44–51. [Google Scholar]
- Deguine J. First encounters. Cell. 2018;175(3):601–603. doi: 10.1016/j.cell.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Demmelmair H., Prell C., Timby N., Lonnerdal B. Benefits of lactoferrin, osteopontin and milk fat globule membranes for infants. Nutrients. 2017;9(8):E817. doi: 10.3390/nu9080817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewettinck K., Rombaut R., Thienpont N., Le T.T., Messens K., Van Camp J. Nutritional and technological aspects of milk fat globule membrane material. Int Dairy J. 2008;18(5):436–437. [Google Scholar]
- Elamin E.E., Masclee A.A., Dekker J., Pieters H.J., Jonkers D.M. Short-chain fatty acids activate AMP-activated protein kinase and ameliorate ethanol-induced intestinal barrier dysfunction in caco-2 cell monolayers. J Nutr. 2013;143(12):1872–1881. doi: 10.3945/jn.113.179549. [DOI] [PubMed] [Google Scholar]
- Grevengoed T.J., Klett E.L., Coleman R.A. Acyl-CoA metabolism and partitioning. Annu Rev Nutr. 2014;34:1–30. doi: 10.1146/annurev-nutr-071813-105541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin J., Soligot C., Burgain J., Huguet M., Francius G., El-Kirat-Chatel S. Adhesive interactions between milk fat globule membrane and Lactobacillus rhamnosus GG inhibit bacterial attachment to caco-2 TC7 intestinal cell. Colloids Surf B Biointerfaces. 2018;167:44–53. doi: 10.1016/j.colsurfb.2018.03.044. [DOI] [PubMed] [Google Scholar]
- Guilloteau P., Zabielski R., Hammon H.M., Metges C.C. Nutritional programming of gastrointestinal tract development. Is the pig a good model for man? Nutr Res Rev. 2010;23(1):4–22. doi: 10.1017/S0954422410000077. [DOI] [PubMed] [Google Scholar]
- Halpern M.D., Denning P.W. The role of intestinal epithelial barrier function in the development of NEC. Tissue Barriers. 2015;3(1–2) doi: 10.1080/21688370.2014.1000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B.B., Bai Y., Jiang L.L., Wang W., Li T.T., Liu P. Effects of oat bran on nutrient digestibility, intestinal microbiota, and inflammatory responses in the hindgut of growing pigs. Int J Mol Sci. 2018;19(8):E2407. doi: 10.3390/ijms19082407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Parenti M., Grip T., Lonnerdal B., Timby N., Domellof M. Fecal microbiome and metabolome of infants fed bovine MFGM supplemented formula or standard formula with breast-fed infants as reference: a randomized controlled trial. Sci Rep. 2019;9(1):11589. doi: 10.1038/s41598-019-47953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernell O., Domellof M., Grip T., Lonnerdal B., Timby N. Physiological effects of feeding infants and young children formula supplemented with milk fat globule membranes. Nestle Nutr Inst Workshop Ser. 2019;90:35–42. doi: 10.1159/000490291. [DOI] [PubMed] [Google Scholar]
- Hotamisligil G.S., Bernlohr D.A. Metabolic functions of FABPs-mechanisms and therapeutic implications. Nat Rev Endocrinol. 2015;11(10):592–595. doi: 10.1038/nrendo.2015.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Wu Z.H., Liu C., Han D.D., Feng C.P., Wang S.L. Milk fat globule membrane supplementation promotes neonatal growth and alleviates inflammation in low-birth-weight mice treated with lipopolysaccharide. BioMed Res Int. 2019:4876078. doi: 10.1155/2019/4876078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerou-Luron I.L., Lemaire M., Blat S. Health benefits of dairy lipids and MFGM in infant formula. OCL. 2018;25(3):D306. [Google Scholar]
- Inoue R., Tsukahara T., Nakanishi N., Ushida K. Development of the intestinal microbiota in the piglet. J Gen Appl Microbiol. 2005;51(4):257–265. doi: 10.2323/jgam.51.257. [DOI] [PubMed] [Google Scholar]
- Iwasaki A., Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015;16(4):343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C.J., Zheng L., Campbell E.L., Saeedi B., Scholz C.C., Bayless A.J. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17(5):662–671. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Xu W.L., Ma Y., Zhou X.B., Xiao R. Milk fat globule membrane protein promotes C2C12 cell proliferation through the PI3K/Akt signaling pathway. Int J Biol Macromol. 2018;114:1305–1314. doi: 10.1016/j.ijbiomac.2018.04.026. [DOI] [PubMed] [Google Scholar]
- Li Y., Wu J., Niu Y., Chen H., Tang Q., Zhong Y. Milk fat globule membrane inhibits NLRP3 inflammasome activation and enhances intestinal barrier function in a rat model of short bowel. J Parenter Enteral Nutr. 2018;43:677–685. doi: 10.1002/jpen.1435. [DOI] [PubMed] [Google Scholar]
- Lin L., Zhang J.Q. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 2017;18(1):1–5. doi: 10.1186/s12865-016-0187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N., Guo P.T., Zhang J., He T., Kim S.W., Zhang G.L. Nutrients mediate intestinal bacteria-mucosal immune crosstalk. Front Immunol. 2018;9:5. doi: 10.3389/fimmu.2018.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott A.J., Huffnagle G.B. The microbiome and regulation of mucosal immunity. Immunology. 2014;142(1):24–31. doi: 10.1111/imm.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuckin M.A., Lindén S.K., Sutton P., Florin T.H. Mucin dynamics and enteric pathogens. Nat Rev Microbiol. 2011;9(4):265–268. doi: 10.1038/nrmicro2538. [DOI] [PubMed] [Google Scholar]
- Mou Q., Yang H.S., Yin Y.L., Huang P.F. Amino acids influencing intestinal development and health of the piglets. Animals (Basel) 2019;9(6):E302. doi: 10.3390/ani9060302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylund L., Satokari R., Salminen S., de Vos W.M. Intestinal microbiota during early life – impact on health and disease. Proc Nutr Soc. 2014;73(4):457–459. doi: 10.1017/S0029665114000627. [DOI] [PubMed] [Google Scholar]
- Park E.J., Thomson A.B., Clandinin M.T. Protection of intestinal occludin tight junction protein by dietary gangliosides in lipopolysaccharide-induced acute inflammation. J Pediatr Gastroenterol Nutr. 2010;50(3):321–328. doi: 10.1097/MPG.0b013e3181ae2ba0. [DOI] [PubMed] [Google Scholar]
- Paturi G., Butts C.A., Hedderley D., Stoklosinski H., Martell S., Dinnan H. Goat and cow milk powder-based diets with or without prebiotics influence gut microbial populations and fermentation products in newly weaned rats. Food Bioscience. 2018;24:73–79. [Google Scholar]
- Pelaseyed T., Bergstrom J.H., Gustafsson J.K., Ermund A., Birchenough G.M., Schutte A. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev. 2014;260(1):8–20. doi: 10.1111/imr.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdijk O., van Baarlen P., Fernandez-Gutierrez M.M., van den Brink E., Schuren F.H.J., Brugman S. Sialyllactose and galactooligosaccharides promote epithelial barrier functioning and distinctly modulate microbiota composition and short chain fatty acid production in vitro. Front Immunol. 2019;10:94. doi: 10.3389/fimmu.2019.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L.S., Ji W., Wang J.L., Li B., Hu J.P., Wu X. Effects of dietary supplementation with yeast glycoprotein on growth performance, intestinal mucosal morphology, immune response and colonic microbiota in weaned piglets. Food Funct. 2019;10(5):2359–2361. doi: 10.1039/c8fo02327a. [DOI] [PubMed] [Google Scholar]
- Ren W.K., Wang P., Yan J.M., Liu G., Zeng B.H., Hussain T. Melatonin alleviates weanling stress in mice: involvement of intestinal microbiota. J Pineal Res. 2018;64(2) doi: 10.1111/jpi.12448. [DOI] [PubMed] [Google Scholar]
- Rowland I., Gibson G., Heinken A., Scott K., Swann J., Thiele I. Gut microbiota functions: Metabolism of nutrients and other food components. Eur J Nutr. 2017;57(1):1–4. doi: 10.1007/s00394-017-1445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber F., Arasteh I.M., Lawley T.D. Pathogen resistance mediated by IL-22 signaling at the epithelial-microbiota interface. J Mol Biol. 2015;427(23):3676–3682. doi: 10.1016/j.jmb.2015.10.013. [DOI] [PubMed] [Google Scholar]
- Singh R., Chandrashekharappa S., Bodduluri S.R., Baby B.V., Hegde B., Kotla N.G. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf 2 pathway. Nat Commun. 2019;10(1):89. doi: 10.1038/s41467-018-07859-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow D.R., Ward R.E., Olsen A., Jimenez-Flores R., Hintze K.L. Membrane-rich milk fat diet provides protection against gastrointestinal leakiness in mice treated with lipopolysaccharide. J Dairy Sci. 2011;94(5):2201–2202. doi: 10.3168/jds.2010-3886. [DOI] [PubMed] [Google Scholar]
- Sonnenberg G.F., Fouser L.A., Artis D. Border patrol: Regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12(5):383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- Sovran B., Loonen L.M., Lu P., Hugenholtz F., Belzer C., Stolte E.H. IL-22-STAT3 pathway plays a key role in the maintenance of ileal homeostasis in mice lacking secreted mucus barrier. Inflamm Bowel Dis. 2015;21(3):531–532. doi: 10.1097/MIB.0000000000000319. [DOI] [PubMed] [Google Scholar]
- Spitsberg V.L. Invited review: Bovine milk fat globule membrane as a potential nutraceutical. J Dairy Sci. 2005;88:2289–2294. doi: 10.3168/jds.S0022-0302(05)72906-4. [DOI] [PubMed] [Google Scholar]
- Sprong R.C., Hulstein M.F., Lambers F.F., van der Meer R. Sweet buttermilk intake reduces colonisation and translocation of Listeria monocytogenes in rats by inhibiting mucosal pathogen adherence. Br J Nutr. 2012;108(11):2026–2033. doi: 10.1017/S0007114512000165. [DOI] [PubMed] [Google Scholar]
- Tamburini S., Shen N., Wu H.C., Clemente J.C. The microbiome in early life: Implications for health outcomes. Nat Med. 2016;22(7):713–722. doi: 10.1038/nm.4142. [DOI] [PubMed] [Google Scholar]
- Teller I.C., Hoyer-Kuhn H., Bronneke H., Nosthoff-Horstmann P., Oosting A., Lippach G. Complex lipid globules in early-life nutrition improve long-term metabolic phenotype in intra-uterine growth-restricted rats. Br J Nutr. 2018;120(7):763–766. doi: 10.1017/S0007114518001988. [DOI] [PubMed] [Google Scholar]
- Timby N., Domellof E., Hernell O., Lonnerdal B., Domellof M. Neurodevelopment, nutrition, and growth until 12 mo of age in infants fed a low-energy, low-protein formula supplemented with bovine milk fat globule membranes: a randomized controlled trial. Am J Clin Nutr. 2014;99(4):860–868. doi: 10.3945/ajcn.113.064295. [DOI] [PubMed] [Google Scholar]
- Valdes A.M., Walter J., Segal E., Spector T.D. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:k2179. doi: 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanabria R., Tellez A.M., Griffiths M., Sharif S., Corredig M. Modulation of immune function by milk fat globule membrane isolates. J Dairy Sci. 2014;97(4):2017–2026. doi: 10.3168/jds.2013-7563. [DOI] [PubMed] [Google Scholar]
- Zhang X.Y., Wu Y.J., Ye H., Feng C.P., Han D.D., Tao S.Y. Dietary milk fat globule membrane supplementation during late gestation increased the growth of neonatal piglets by improving their plasma parameters, intestinal barriers, and fecal microbiota. RSC Adv. 2020;10(29):16987–16988. doi: 10.1039/d0ra02618b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.