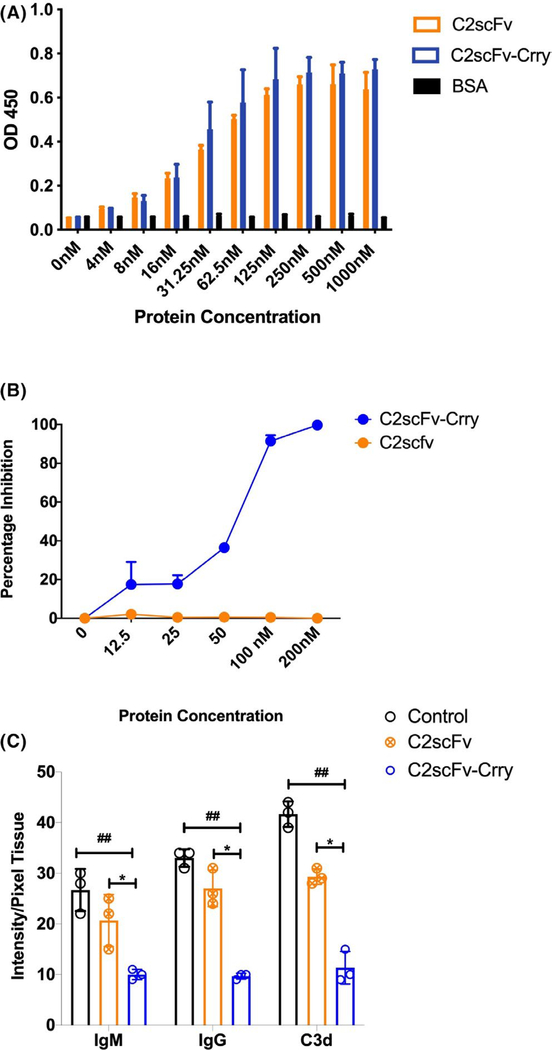
FIGURE 2.
In vitro characterization of C2scFv and C2scFv-Crry. A. Phospholipid binding. Binding of C2scFv and C2scFv-Crry to cardiolipin-coated ELISA plate wells, a known ligand for C2 nAb IgM. BSA coated wells were used for specificity control. Mean ± SEM, n = 3. B. Complement inhibitor activity. Complement inhibitory activity of C2scFv and C2scFv-Crry as measured by zymosan assay. Increasing concentrations of the constructs were incubated with activated zymosan particles in mouse serum, and C3 deposition assayed by flow cytometry. Mean ± SD, n = 3. C. Ab and C3 cellular deposition after injury. Effect of C2scFv and C2scFv-Crry on IgM, IgG and C3d deposition on endothelial cells under conditions of simulated cold storage and reperfusion with pooled mouse serum. C2scFv-Crry significantly reduced binding of all molecules. ##p < .01 C2scFv-Crry vs Control and *p < .01 C2scFv-Crry vs C2scFv. Representative of n = 3 experiments