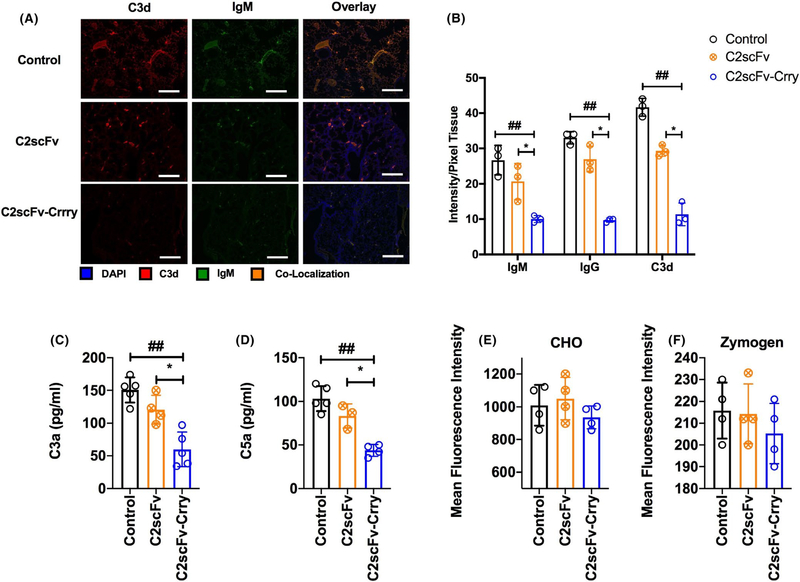
FIGURE 4.
C2scFv-Crry reduces IgM and complement deposition in transplanted lungs with minimal impact on systemic complement activity. All analyses at 48 h after transplant. A. Immunofluorescent localization of IgM, IgG and C3d in transplanted lungs. Representative images, n = 3. Scale bar 100 μm. B. Quantification of IgM, IgG and C3d by pixel fluorescence intensity normalized to tissue area. C2scFv-Crry treatment of recipients significantly reduced deposition of all molecules in transplanted lungs relative PBS and C2scFv treatment. For IgM, ##PBS control vs. C2scFv-Crry (p = .04), and *C2scFv vs. C2scFv-Crry (p = .01); for IgG, ##PBS control vs. C2scFv-Crry (p = .01), and*C2scFv vs. C2scFv-Crry (p = .001), and C3d; ##PBS control vs. C2scFv-Crry (p = .03), and*C2scFv vs. C2scFv-Crry (p = .01). No significant difference was seen between IgM, IgG, or C3d deposition in lungs from PBS- vs C2scFv-treated mice. Mean ± SEM, n = 3. C and D. C2scFv-Crry treatment significantly reduced lung allograft C3a and C5a levels as compared to PBS and C2scFv treatment. For C3a, ##PBS control vs. C2scFv-Crry (p = .002), and *C2scFv vs. C2scFv-Crry (p = .002); for C5a, ##PBS control vs. C2scFv-Crry (p = .01), and *C2scFv vs. C2scFv-Crry (p = .005). E and F. C2scFv-Crry did not significantly impact systemic complement activity as determined by CHO cell lysis assay (E) and zymozan C3d deposition assay (F), which measure classical and alternative pathway activity, respectively