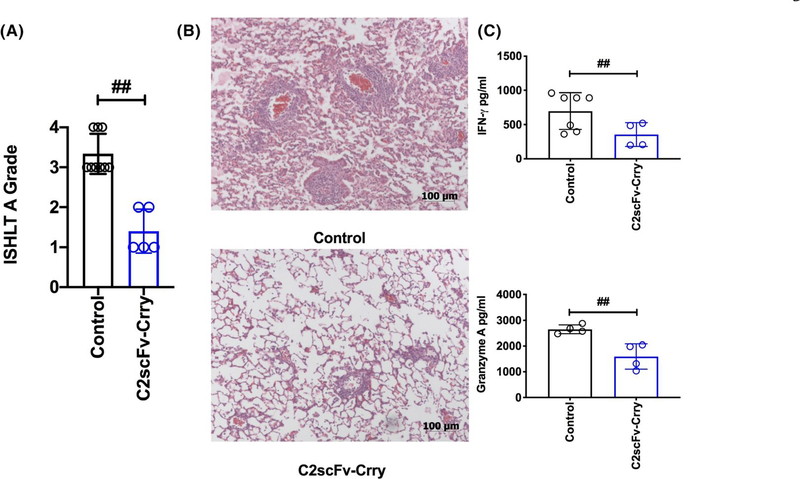
FIGURE 6.
C2scFv-Crry ameliorates acute cellular rejection measured at day 7 posttransplant. A. Histopathological lung graft analysis (refer to methods for scoring system). All graft samples demonstrated characteristic perivascular immune infiltrates, but pairwise comparisons of ISHLT A grades demonstrated that acute rejection is significantly reduced in C2scFv-Crry-treated recipients. ##PBS control vs. C2scFv-Crry (p = .0005). B. Representative hematoxylin and eosin stained lung sections, depicting data shown in (A). C. Splenic immune cells from each recipient were isolated at 7 days posttransplant and co-incubated with donor splenocytes. The mixed lymphocyte reactions show a significant reduction in systemic allogenicity as demonstrated by a reduction in T cell IFNγ and Granzyme A production in C2scFv-Crry at day 7 posttransplant. IFNγ ##PBS control vs. C2scFv-Crry (p = .048), and Granzyme A ##PBS control vs. C2scFv-Crry (p = .006)