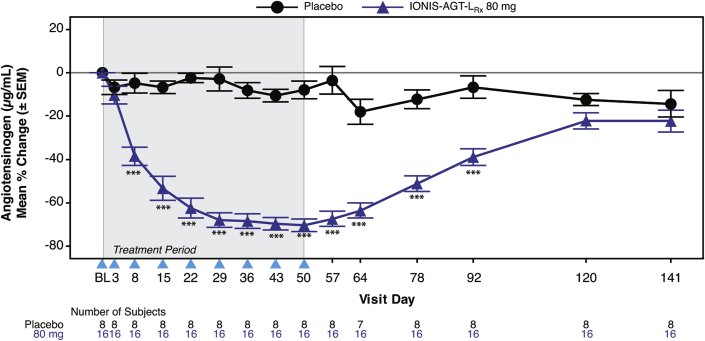
Figure 2.
Mean Percent Changes in AGT Over Time in IONIS-AGT-LRx Versus Placebo in the Add-On Study
The shaded area represents the dosing window, the arrowheads show the timepoint when the dose was given, and the unshaded area shows the follow-up period. The primary endpoint was at day 57. ∗p < 0.05; ∗∗p < 0.01, ∗∗∗p < 0.001 IONIS-AGT-LRx versus placebo at the specified timepoint. Abbreviation as in Figure 1.