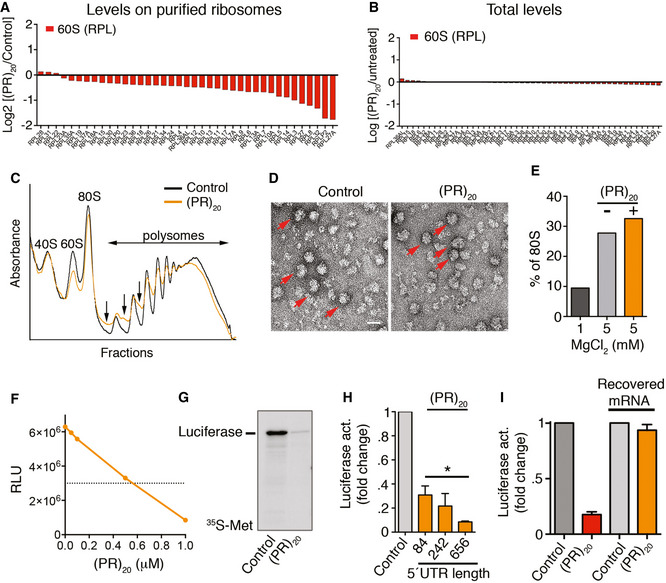
Protein levels of RPL factors in ribosomes purified from Hela RPS9SBP cells exposed to 10 μM of (PR)20 for 16 h, as identified by LC‐MS/MS.
Protein levels of RPL factors in the input extracts used for ribosome purification from Hela RPS9SBP cells exposed to 10 μM of (PR)20 for 16 h, as identified by LC‐ MS/MS.
Representative polysome profiles obtained from HeLa cells untreated or treated with 10 μM of (PR)20 for 16 h. The presence of halfmers is indicated (arrows).
Electron microscopy images from purified 40S and 60S ribosomal complexes (1 pmol each) assembled in vitro in the presence of MgCl2, and in the presence or absence of 5 pmol of (PR)20. Assembled 80S particles are indicated (red arrows). Scale bar (white) represents 10 nm.
Quantification of 80S particles identified in (D) (n = 1,000) in non‐assembly (1 mM MgCl2) or assembly (5 mM MgCl2) conditions.
In vitro translation of 100 ng of luciferase mRNA (quantified by Relative Luciferase Units [RLU]) in the presence of increasing doses of (PR)20.
In vitro translation of 100 ng of luciferase mRNA in the presence or absence of 0.5 μM (PR)20. Translation products were labeled with [35S]‐Met/Cys and analyzed by SDS–PAGE and autoradiography.
In vitro translation of 100 ng of luciferase mRNA with different 5′ UTR lengths in the presence (orange columns) or absence (grey column) of 0.5 μM (PR)20 (n = 3). Data represent mean values ± SD (*P < 0.05; t‐test).
In vitro translation of 100 ng of luciferase mRNA in the presence or absence of 0.5 μM (PR)20. In the right two columns, the mRNA was extracted from a translation reaction done in the presence of the DPR, and subsequently used in a new translation reaction performed in the absence of (PR)20 (n = 3). Data represent mean values ± SD.