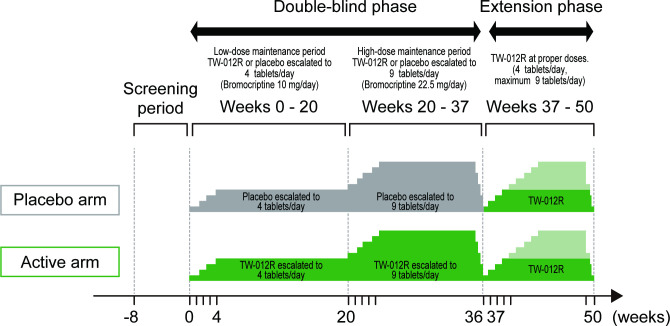
Figure 1.
Design of REBRAnD study. The study consists of the screening period (8 weeks), double-blind phase (37 weeks) and extension phase (13 weeks). The double-blind phase for evaluating the efficacy and safety is composed of the low-dose maintenance period (up to 10 mg/day), high-dose maintenance period (up to 22.5 mg/day) and tapering period of the trial drug. Additionally, there is an open-labelled active drug extension period (up to 10 or 22.5 mg/day) for evaluating long-term safety.