ABSTRACT
Background
Research has established that maternal diet influences fetal growth and preterm birth, but most studies only evaluate single nutrients. Relations between dietary patterns and neonatal outcomes are understudied.
Objective
We evaluated associations of neonatal outcomes with maternal diet patterns derived using 3 a priori diet scores [Alternative Healthy Eating Index-2010 (AHEI-2010), alternate Mediterranean diet score (aMed), and Dietary Approaches to Stop Hypertension (DASH)] as well as principal components analysis (PCA).
Methods
We studied 1948 women from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Fetal Growth Studies-Singletons, a racially diverse multisite cohort of pregnant women in the USA (2009–2013). Diet in the past 3 mo was assessed using a self-administered FFQ at 8–13 weeks of gestation. Birthweight was abstracted from medical records and neonatal anthropometry measured postdelivery using standardized protocols.
Results
All 3 a priori scores were significantly associated with increased birthweight, and aMed was also associated with reduced odds of low birthweight [quartile 4 versus 1: ORadj = 0.42; 95% CI: 0.18, 1.00 (P-trend = 0.02)]. Greater aMed and DASH scores were significantly associated with increased length [aMed: quartile 4 versus 1: 0.54 cm; 95% CI: 0.10, 0.99 (P-trend = 0.006); DASH: quartile 4 versus 1: 0.62 cm; 95% CI: 0.25, 0.99 (P-trend = 0.006)] and upper arm length. Neither diet pattern derived from PCA was significantly associated with birthweight.
Conclusion
Among mostly low-risk pregnant women, pre- and early pregnancy healthful diet quality indices, particularly the aMed score, were associated with larger neonatal size across the entire birthweight distribution. In the absence of generally accepted pregnancy-specific diet quality scores, these results provide evidence for an association between maternal diet patterns and neonatal outcomes.
Keywords: maternal diet patterns, a priori scores, principal components analysis, neonatal anthropometry, preterm birth, prospective cohort
Introduction
Suboptimal fetal growth has short- and long-term implications for the health of offspring (1, 2). Maternal diet is a potentially modifiable risk factor that can influence neonatal size. Many available studies on the role of maternal diet and neonatal anthropometric outcomes employ a single-nutrient approach (3, 4). Yet public health recommendations based on single nutrients are less intuitive and translatable, given individuals eat meals with complex compositions of food groups and constituent nutrients (5–7).
The shortcomings of the single-nutrient approach can be addressed in 2 primary ways. One option is to calculate a composite index score based on a set of a priori criteria of diet quality (8–11). In particular, the Alternative Healthy Eating Index-2010 (AHEI-2010), the alternate Mediterranean diet score (aMed), and Dietary Approaches to Stop Hypertension (DASH) are widely used indices originally developed to reflect associations with decreased chronic disease outcomes and improved survival (10–13). Another option is to employ methods such as principal components analysis (PCA), to identify diet patterns in terms of common combinations of food intake observed within the data for a given sample. Previous studies on the relation between maternal diet and neonatal outcomes have relied on these approaches (14). This research, however, exhibits notable limitations. Some studies were conducted in cohorts of mostly white women, despite the evidence that dietary patterns are influenced by race/ethnicity (15, 16). Also, many studies evaluate only 1 of the current a priori scores (17, 18) though determining which of them is most relevant in pregnancy may help inform a standardized way to define diet quality for policies and programs related to maternal and child health (19).
Therefore, our objective was to comprehensively evaluate the association of early pregnancy maternal diet using both a priori scores and PCA-derived patterns, with neonatal outcomes among a contemporary multiethnic cohort of US pregnant women. The primary outcome was birthweight, along with more clinically meaningful outcomes like small- and large-for-gestational age. We further examined associations with measures of neonatal anthropometry (length, upper arm length, upper thigh length, head circumference, abdominal circumference, sum of skinfold thickness) which are useful in understanding the mechanism by which birthweight may be affected, as well as for observing suboptimal anthropometric phenotypes that can occur in the presence of normal birthweight. Lastly, we examined associations with preterm delivery, which influences anthropometry, but is also independently a critical neonatal outcome.
Methods
Study population
This analysis was a secondary analysis of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Fetal Growth Studies-Singletons (2009–2013) which was a prospective cohort study of women with singleton pregnancies with a primary aim of establishing standards for fetal growth (20). Details of the cohort are described elsewhere (21). In brief, 2802 non-Hispanic white, non-Hispanic black, Hispanic, and Asian/Pacific Islander women were recruited from 12 clinical sites around the USA. By design, 468 of the women had obesity, and the remaining nonobese women were nonsmokers and free of chronic diseases. Women were enrolled at 8–13 weeks of gestation. Participants were followed up for a maximum of 5 additional visits throughout their pregnancy and postdelivery. The study was approved by the Institutional Review Boards of the NICHD, the data coordinating centers, and clinical sites. All participants signed informed consent forms prior to enrollment.
Dietary assessment
Dietary intake was assessed at enrollment (8–13 weeks of gestation). A modified version of the Diet History Questionnaire-2 (DHQ-2) FFQ was used to capture diet in the past 3 mo (i.e., first trimester). Using the Diet*Calc software, we calculated the daily intake of 176 nutrients, diet constituents, and food groups based on participants’ responses. Dietary data that was considered implausible [daily total energy <600 kcal/d or >6000 kcal/d (22)] were set to missing and later imputed (n = 74). A priori and PCA diet pattern scores were imputed at the score level.
The AHEI-2010 includes 11 components of food groups and nutrients. The scoring criteria for each component are detailed elsewhere (10). Briefly, higher intakes of vegetables, fruits, whole grains, nuts and legumes, long-chain [ω-3 (n–3)] fatty acids (EPA and DHA), PUFAs, and moderate alcohol count favorably, whereas higher intakes of sugar-sweetened beverages, red/processed meats, trans fat, and sodium count unfavorably. Each component of the score is rated on a scale of 0 to 10, with higher scores being indicative of better diet quality. We modified the AHEI-2010 by eliminating the alcohol component, which is unsuitable for pregnancy. The scores for the adjusted index therefore range from 0 to 100.
The aMed is a version of the Mediterranean diet score designed by Trichopoulou et al. (23), which is modified to be more applicable to US diets (11). The 9 components are: vegetables excluding potatoes; legumes; fruit; nuts; whole grains; red and processed meats; fish; ratio of monounsaturated to saturated fat; and ethanol. Each component is assigned either 0 or 1 point; meat consumption less than the median is assigned a 1, whereas consumption above the median for the other components was assigned a 1. Here too, we adjusted the aMed by eliminating the alcohol component. The score for the adjusted aMed therefore ranges from 0 to 8.
DASH is an 8-component diet quality index designed by Fung et al. (24). Scoring is based on quintiles of intake. The highest quintiles of red and processed meat, sugar-sweetened beverages, and sodium are given a score of 1, with their lowest quintiles given a score of 5. Conversely, the highest quintiles of vegetables, fruits, whole grains, low-fat dairy, nuts, seeds, and legumes are given a score of 5, with the lowest quintiles given a 1. The total score can range from 8 to 40.
For the derivation of the PCA-based patterns, we used input variables in the form of My Pyramid Equivalent Database (MPED) serving units, a food-grouping system developed by the USDA that disaggregates the ingredients of foods into major groups and subgroups (25). After excluding alcohol, we retained 26 input variables: whole grain; refined grain; meat from beef, pork, veal, lamb, and game; meat from organ meat foods; cured meat; poultry; seafood high in ω-3; seafood low in ω-3; soy products; nuts and seeds; legumes; dark-green vegetable; red/orange tomato vegetable; red/orange other vegetable; white potato starchy vegetable; other starchy vegetable; other vegetable; citrus, melon, and berry fruit; other fruit; milk; eggs; yogurt; cheese; oil; solid fat; and added sugar. The ranges of these input variables differed considerably. Therefore, we standardized each input variable prior to performing PCA, by subtracting the mean and dividing by the SD (26).
Covariate data
A research nurse interviewed each participating woman at enrollment to collect data on age, race, income, education, employment (or student) status, marital status, insurance coverage, and reproductive history. Each participating woman also completed a validated pre- and periconception physical activity questionnaire from which total physical activity [metabolic equivalent of task (MET) hours per week] was assessed (27). Prepregnancy BMI was calculated from measured height and self-reported prepregnancy weight. The correlation between measured and abstracted maternal weights in our study was very high (r = 0.998) (28). Offspring sex was determined at birth.
Outcome data
Data were collected on several neonatal outcomes. Birthweight was abstracted from medical charts. Other anthropometric measures (length, upper arm length, upper thigh length, head circumference, abdominal circumference, sum of skinfold thickness) were recorded by trained research nurses postdelivery using standardized protocols (29–32). Birthweight was categorized into normal weight, low birthweight (LBW) (<2500 g), and macrosomia (≥4000 g). Birthweight and gestational age at delivery were used together to categorize each neonate as small-for-gestational age (SGA), appropriate-for-gestational age (AGA), or large-for-gestational age (LGA), using a sex-specific US reference (33). Gestational age was calculated from the self-reported known last menstrual period date, confirmed through a first-trimester ultrasound. We defined preterm birth as births <37 weeks of gestation.
Statistical analysis
To derive diet patterns based on observed consumption, the 26 food group input variables were standardized and entered into a PCA procedure with orthogonal rotation in SAS (SAS Institute). Scree plots, eigenvalues, absolute factor loadings, and interpretability were used to guide selection of diet patterns. To assess the internal validity of diet patterns, in terms of the stability of factor solutions (34–37), we randomly split the analytic sample into 2 and performed PCA again. Selected PCA diet patterns as well as the AHEI-2010, aMed, and DASH scores calculated according to the aforementioned criteria were ranked into quartiles, for consistency with previous literature and comparability across scores. We examined agreement among quartiles of diet patterns using weighted κ-coefficients.
Descriptive characteristics of participants across quartiles of each diet pattern were compared using ANOVA and chi-squared tests as appropriate. With the lowest quartile of each diet pattern as referent, ORs from logistic regression were estimated for categorical outcomes. β-coefficients from linear regression were estimated for continuous outcomes. We also examined linear P-trends for each diet pattern and outcome using the median diet scores at each quartile. To control for multiple comparisons, we also indicated which significant P-trends survive false discovery rate (FDR) adjustment. Models of neonatal outcomes were not adjusted for gestational age, which may be in the causal pathway, leading to a potential for inducing bias if included (38).
We examined the independent association of each dietary pattern with neonatal outcomes in adjusted models that controlled for maternal age, height, parity, prepregnancy BMI, race, marital status, education, income, current employment/student status, insurance coverage, infant sex, total weekly physical activity, total daily energy intake, and study site. When the outcome was length, upper arm length, upper thigh length, head circumference, abdominal circumference, or skinfold thickness, models also adjusted for the infant's age at the postnatal measurement. We addressed missing exposure, covariate, and outcome data with 20 iterations of multiple imputation, assuming values were missing at random (39). In a sensitivity analysis to address the less strict inclusion criteria among women with obesity, we repeated the analyses after excluding women with obesity who reported smoking (n = 17).
All analyses were performed in SAS 9.4 (SAS Institute).
Results
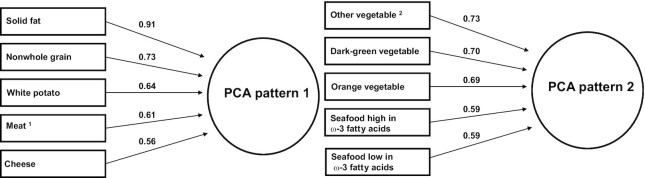
The analytic sample included 1948 women with a live birth (Supplemental Figure 1). The 2 major diet patterns derived via PCA and the top 5 food groups represented are shown in Figure 1. PCA pattern 1 (eigenvalue = 5.51; variance explained = 21.2%), was characterized by higher intakes of solid fat, nonwhole grains, white potatoes, meat (from beef, pork, veal, lamb, and game), and cheese. In contrast, PCA pattern 2 (eigenvalue = 2.59; variance explained = 10.0%) was characterized by higher intakes of other vegetables (not potatoes, starchy, orange, or dark-green vegetables), dark-green vegetables, orange vegetables, seafood high in ω-3 fatty acids, and seafood low in ω-3 fatty acids. Factor loadings of all 26 input food groups for these 2 diet patterns are presented in Supplemental Table 1. When randomly splitting the analytic sample into 2 subsamples to assess the internal validity of diet patterns, the top PCA-derived patterns in 1 set remained similar (in terms of variance explained, eigenvalue, and top food groups that loaded onto each pattern) to those of the other set, as well as to those derived from the entire sample, indicating the findings were robust (data not shown). Agreement among diet patterns is reported in Supplemental Table 2. Weighted κ statistics for agreement between PCA pattern 1 and all other patterns were negative or <0.06. PCA pattern 2 had fair agreement with AHEI-2010 and DASH, and moderate agreement with aMed. Agreements of a priori scores with each other were all in the moderate (0.43–0.47) range (40).
FIGURE 1.
Factor loadings for the 2 major principal components analysis-derived diet patterns, NICHD Fetal Growth Studies-Singletons, 2009–2013. 1Meat from beef, pork, veal, lamb, and game (excludes organ meat). 2Vegetable other than dark-green vegetable, orange vegetable, white potato, and other starchy vegetable. NICHD, National Institute of Child Health and Human Development; PCA, principal components analysis.
Across increasing quartiles of PCA pattern 1, women were significantly younger, less educated, of lower income, more likely to be non-Hispanic black, less likely to have private insurance, and of higher mean prepregnancy BMI (Table 1). These distributions were reversed for PCA pattern 2 and all 3 a priori diet indices. Women also had a higher mean intake of total energy, and shares of energy from protein and fats with increasing quartiles of PCA pattern 1. Across increasing quartiles of PCA pattern 2 and all 3 a priori diet indices, women had lower mean intakes of total energy and shares of energy from protein. Across increasing quartiles of PCA pattern 2 and AHEI-2010, women also had lower shares of energy from carbohydrates, but a higher share of energy from fats. Women in higher quartiles of the DASH score had a higher share of energy from carbohydrates, but a lower share of energy from fats.
TABLE 1.
Description of cohort by quartiles of principal components analysis-derived and a priori diet patterns, NICHD Fetal Growth Studies-Singletons, 2009–20131
| PCA pattern 1 | PCA pattern 2 | AHEI-2010 | aMED | DASH | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q4 | Q1 | Q4 | Q1 | Q4 | Q1 | Q4 | Q1 | Q4 | |
| Range | (−1.7, −0.5) | (0.2, 3.0) | (−1.5, −0.6) | (0.3, 6.2) | (22.9, 44.6) | (58.1, 80.1) | (0, 2) | (6, 8) | (11, 20) | (28, 37) |
| N | 400 | 400 | 400 | 400 | 400 | 400 | 351 | 336 | 354 | 360 |
| Demographics | ||||||||||
| Age, y | 29.5 ± 5.4 | 25.4 ± 5.4*** | 25.4 ± 5.3 | 29.7 ± 5.3*** | 25.5 ± 5.5 | 30.7 ± 4.7*** | 26.4 ± 5.7 | 29.9 ± 5.3*** | 25.6 ± 5.4 | 30.4 ± 4.8*** |
| Race | ||||||||||
| Non-Hispanic white | 65 (16) | 52 (13)*** | 64 (16) | 76 (19)*** | 57 (14) | 112 (28)*** | 73 (21) | 87 (26)*** | 36 (10) | 135 (38)*** |
| Non-Hispanic black | 77 (19) | 200 (50) | 187 (47) | 77 (19) | 214 (54) | 55 (14) | 140 (40) | 76 (23) | 195 (55) | 51 (14) |
| Hispanic | 116 (29) | 115 (29) | 119 (30) | 98 (25) | 98 (25) | 102 (26) | 100 (28) | 84 (25) | 76 (22) | 104 (29) |
| Asian/Pacific Islander | 142 (36) | 33 (8) | 30 (8) | 149 (37) | 31 (8) | 131 (33) | 38 (11) | 89 (26) | 47 (13) | 70 (19) |
| Married | 323 (81) | 232 (58) | 242 (61) | 332 (83)*** | 220 (55) | 351 (88)*** | 233 (67) | 270 (80)*** | 193 (55) | 309 (86)*** |
| Socioeconomic status | ||||||||||
| Education | ||||||||||
| <High school | 50 (13) | 61 (15)*** | 58 (15) | 42 (11)*** | 58 (14) | 35 (9)*** | 50 (14) | 28 (8)*** | 53 (15) | 31 (9)*** |
| High school or equivalent | 61 (15) | 117 (29) | 108 (27) | 62 (16) | 121 (30) | 48 (12) | 98 (18) | 41 (12) | 110 (31) | 37 (10) |
| Some college/associate | 117 (29) | 133 (33) | 155 (39) | 100 (25) | 143 (36) | 85 (21) | 122 (35) | 89 (27) | 127 (36) | 86 (24) |
| College undergraduate | 98 (25) | 55 (14) | 58 (15) | 103 (26) | 51 (13) | 113 (28) | 49 (14) | 90 (27) | 44 (12) | 95 (26) |
| Postgraduate | 74 (19) | 34 (9) | 21 (5) | 93 (23) | 27 (7) | 119 (30) | 32 (9) | 88 (26) | 20 (6) | 111 (31) |
| Income, thousands (US dollars) | ||||||||||
| <30 | 92 (28) | 170 (50)*** | 151 (45) | 91 (28)*** | 168 (50) | 73 (22)*** | 119 (40) | 66 (24)*** | 155 (54) | 55 (18)*** |
| 30–39 | 33 (10) | 26 (8) | 40 (12) | 33 (10) | 37 (11) | 21 (6) | 40 (13) | 13 (5) | 27 (9) | 24 (8) |
| 40–49 | 28 (9) | 32 (9) | 36 (11) | 23 (7) | 31 (9) | 21 (6) | 24 (8) | 18 (6) | 27 (9) | 19 (6) |
| 50–75 | 52 (16) | 38 (11) | 40 (12) | 38 (12) | 36 (11) | 42 (13) | 39 (13) | 37 (13) | 30 (10) | 37 (12) |
| 75–99 | 40 (12) | 24 (7) | 30 (9) | 42 (13) | 29 (9) | 44 (13) | 36 (12) | 38 (14) | 25 (9) | 41 (13) |
| ≥100 | 79 (24) | 48 (14) | 40 (12) | 103 (31) | 35 (10) | 133 (40) | 39 (13) | 108 (39) | 26 (9.0) | 135 (43) |
| Full-time school or work | 260 (65) | 272 (68) | 272 (68) | 269 (67) | 274 (69) | 278 (70) | 231 (66) | 239 (71) | 231 (65) | 268 (74) |
| Insurance (private/managed care) | 256 (66) | 166 (43)*** | 178 (46) | 265 (68)*** | 182 (47) | 294 (76)*** | 184 (54) | 234 (72)*** | 158 (46) | 248 (73)*** |
| Reproductive health | ||||||||||
| Parity | ||||||||||
| 0 | 178 (45) | 194 (49) | 195 (49) | 178 (45)** | 194 (49) | 190 (48) | 150 (43) | 165 (49) | 162 (46) | 180 (50) |
| 1 | 152 (38) | 128 (32) | 131 (33) | 152 (38) | 124 (31) | 152 (38) | 132 (38) | 117 (35) | 118 (33) | 125 (35) |
| 2 | 46 (12) | 44 (11) | 57 (14) | 38 (10) | 57 (14) | 38 (10) | 55 (16) | 32 (10) | 50 (14) | 36 (10) |
| 3 | 17 (4) | 30 (8) | 9 (2) | 26 (7) | 15 (4) | 18 (5) | 6 (2) | 20 (6) | 17 (5) | 14 (4) |
| 4+ | 7 (2) | 4 (1) | 8 (2) | 6 (2) | 10 (3) | 2 (0.5) | 8 (2) | 2 (0.60) | 7 (2) | 5 (1) |
| Maternal lifestyle factors | ||||||||||
| Maternal height, cm | 161.5 ± 6.6 | 162.7 ± 7.1* | 163.0 ± 6.7 | 162.2 ± 6.9 | 162.9 ± 6.9 | 162.4 ± 7.0 | 162.5 ± 7.1 | 162.8 ± 7.5 | 162.5 ± 6.8 | 163.1 ± 7.2 |
| Prepregnancy BMI, kg/m2 | 24.8 ± 5.0 | 25.5 ± 5.1*** | 26.3 ± 5.3 | 24.2 ± 4.6*** | 26.0 ± 5.5 | 24.2 ± 4.4*** | 26.3 ± 5.2 | 24.0 ± 4.0*** | 25.6 ± 5.3 | 24.5 ± 4.4** |
| Daily total energy,2 kcal/d | 1324.4 ± 512.9 | 3492.9 ± 954.8*** | 1860.2 ± 1073.3 | 2635.5 ± 1001.7*** | 2347.3 ± 1203.2 | 1983.1 ± 762.9*** | 1667.9 ± 823.4 | 2543.9 ± 989.0*** | 2251.3 ± 1151.7 | 2239.2 ± 884.3* |
| Carbohydrates,2 % energy | 54.2 ± 8.9 | 54.2 ± 10.6 | 57.1 ± 10.8 | 51.6 ± 8.2*** | 54.5 ± 10.7 | 51.8 ± 7.9*** | 53.2 ± 10.10 | 53.0 ± 7.6 | 52.5 ± 11.0 | 55.4 ± 7.6*** |
| Protein,2 % energy | 16.7 ± 3.6 | 14.7 ± 3.6*** | 13.5 ± 3.4 | 17.7 ± 3.1*** | 14.4 ± 3.7 | 17.3 ± 3.0*** | 15.0 ± 3.6 | 16.9 ± 3.1*** | 14.7 ± 3.8 | 16.6 ± 2.9*** |
| Fat,2 % energy | 31.4 ± 6.7 | 33.0 ± 7.7** | 31.3 ± 8.0 | 33.1 ± 6.7** | 32.6 ± 7.6 | 33.3 ± 6.6*** | 33.5 ± 7.4 | 32.6 ± 5.9 | 34.1 ± 7.9 | 30.9 ± 6.0*** |
| Physical activity, MET h/wk | 292.7 ± 138.6 | 369.2 ± 192.7*** | 325.0 ± 171.0 | 332.5 ± 166.5 | 331.8 ± 171.4 | 304.6 ± 141.4 | 320.2 ± 161.7 | 334.6 ± 167.3 | 325.1 ± 172.2 | 329.2 ± 154.5 |
| Neonatal Factors | ||||||||||
| Male offspring | 206 (54) | 177 (47) | 203 (52) | 205 (53) | 182 (47) | 203 (53) | 166 (49) | 157 (49) | 168 (49) | 168 (49) |
| Measurement date3 | 2.0 ± 4.2 | 1.7 ± 2.8 | 1.7 ± 2.5 | 1.9 ± 4.0 | 1.6 ± 2.7 | 2.0 ± 4.2 | 1.6 ± 2.5 | 1.7 ± 4.0 | 1.6 ± 2.5 | 1.7 ± 4.0 |
Values represent means ± SDs or n (%). *P < 0.05; **P < 0.01; ***P < 0.001 (P values were derived using ANOVA for continuous and chi-squared test for categorical variables, comparing all 4 quartiles). Missing data: n = 254 for income, n = 11 for height, n = 2 for physical activity, n = 1 for marital status, n = 49 for insurance, and n = 60 for infant sex. AHEI-2010, Alternative Healthy Eating Index-2010; aMED, alternative Mediterranean diet score; DASH, Dietary Approaches to Stop Hypertension; MET, Metabolic Equivalents of Task; NICHD, National Institute of Child Health and Human Development; PCA, principal components analysis; Q, quartile.
Nutrient intakes derived from FFQ data.
Measurement date indicates number of days between birth and date of assessment for neonatal anthropometry measurements other than birthweight.
The number of values imputed for each variable used in examining the associations between diet patterns and neonatal outcomes are reported in Supplemental Table 3.
Associations between PCA-derived and a priori diet patterns with continuous and categorical birthweight adjusted for potential confounders are presented in Table 2. PCA patterns 1 and 2 were not significantly associated with any birthweight outcomes. Greater a priori scores were associated with greater birthweight [AHEI: quartile 4 versus 1: 96.56 g; 95% CI: 17.01, 176.11 g (P-trend = 0.039); aMED: quartile 4 versus 1: 125.90 g; 95% CI: 41.79, 210.01 g (P-trend = 0.0021); DASH: quartile 4 versus 1: 100.37 g; 95% CI: 24.95, 175.79 g (P-trend = 0.015)], with the P-trends for aMed and DASH remaining significant after FDR adjustment. The aMed score was associated with reduced odds of LBW [quartile 4 versus 1: ORadj = 0.42; 95% CI: 0.18, 1.00 (P-trend = 0.024, no longer significant after FDR-adjustment)]. Some quartiles of aMed were associated with macrosomia (quartile 3 versus 1: ORadj = 1.89; 95% CI: 1.02, 3.48, P-trend = 0.063), and some specific quartiles of the AHEI and aMed were associated with higher odds of LGA. Adjusted associations of quartiles of PCA-derived and a priori diet patterns with neonatal anthropometry and preterm birth are presented in Table 3. Greater aMed and DASH scores were associated with increased birth length and upper arm length, with significant P-trends even after FDR adjustment. There were no significant associations between diet and preterm birth.
TABLE 2.
Associations of maternal diet patterns with birthweight outcomes, NICHD Fetal Growth Studies-Singletons, 2009–20131
| PCA-derived patterns | A priori patterns | |||||
|---|---|---|---|---|---|---|
| β2 (95% CI) | β2 (95% CI) | |||||
| Continuous outcome | PCA pattern 1 | PCA pattern 2 | AHEI-2010 | aMED | DASH | |
| Birthweight, g | Q1 | Referent | Referent | Referent | Referent | Referent |
| Q2 | −37.82 (−112.96, 37.33) | −21.22 (−98.68, 56.23) | 66.67 (−4.79,138.11) | 13.04 (−64.17, 90.26) | 15.70 (−52.97, 84.37) | |
| Q3 | −65.69 (−148.10, 16.72) | 24.19 (−58.65, 107.03) | 58.70 (−18.94, 136.34) | 65.92 (−5.51, 137.34) | 62.31 (−4.71, 129.34) | |
| Q4 | −52.29 (−177.74, 73.16) | 37.30 (−49.55, 124.15) | 96.56 (17.01, 176.11) | 125.90 (41.79, 210.01) | 100.37 (24.95, 175.79) | |
| P-trend | 0.41 | 0.25 | 0.039 | 0.0021* | 0.015* | |
| OR3 (95% CI) | OR3 (95% CI) | |||||
| Categorical outcomes, % | PCA pattern 1 | PCA pattern 2 | AHEI-2010 | aMED | DASH | |
| Size for gestational age | ||||||
| SGA, 9.1% | Q1 | Referent | Referent | Referent | Referent | Referent |
| Q2 | 1.56 (0.93, 2.67) | 0.70 (0.42, 1.19) | 0.76 (0.47, 1.25) | 1.22 (0.71, 2.11) | 0.85 (0.50, 1.46) | |
| Q3 | 1.37 (0.76, 2.48) | 0.72 (0.41, 1.25) | 0.80 (0.48, 1.31) | 0.89 (0.53, 1.51) | 0.86 (0.51, 1.45) | |
| Q4 | 1.24 (0.53, 2.86) | 0.66 (0.36, 1.21) | 0.55 (0.30, 1.01) | 0.60 (0.29, 1.22) | 0.78 (0.43, 1.43) | |
| P-trend | 0.81 | 0.25 | 0.11 | 0.12 | 0.43 | |
| LGA, 8.0% | Q1 | Referent | Referent | Referent | Referent | Referent |
| Q2 | 0.91 (0.53, 1.56) | 0.81 (0.46, 1.42) | 1.73 (1.02, 2.92) | 1.53 (0.84, 1.51) | 1.29 (0.68, 2.46) | |
| Q3 | 0.92 (0.50, 1.68) | 1.12 (0.64, 1.94) | 1.18 (0.67, 2.07) | 1.81 (1.03, 3.19) | 1.75 (0.95, 3.23) | |
| Q4 | 0.71 (0.28, 1.85) | 0.97 (0.50, 1.88) | 0.91 (0.48, 1.72) | 1.66 (0.85, 3.25) | 1.73 (0.88, 3.40) | |
| P-trend | 0.51 | 0.88 | 0.85 | 0.14 | 0.094 | |
| Birthweight | ||||||
| LBW, 5.5% | Q1 | Referent | Referent | Referent | Referent | Referent |
| Q2 | 1.07 (0.49, 2.34) | 1.08 (0.58, 2.02) | 0.73 (0.39, 1.38) | 0.89 (0.47, 1.69) | 1.15 (0.63, 2.12) | |
| Q3 | 1.47 (0.67, 3.20) | 0.79 (0.37, 1.67) | 0.64 (0.33, 1.22) | 0.49 (0.25, 0.95) | 0.73 (0.38, 1.40) | |
| Q4 | 1.11 (0.34, 3.46) | 0.64 (0.29, 1.41) | 0.48 (0.22, 1.06) | 0.42 (0.18, 1.00) | 0.76 (0.34, 1.69) | |
| P-trend | 0.85 | 0.19 | 0.065 | 0.024 | 0.33 | |
| Macrosomia, 7.1% | Q1 | Referent | Referent | Referent | Referent | Referent |
| Q2 | 1.39 (0.78, 2.48) | 0.95 (0.53, 1.70) | 1.31 (0.75, 2.27) | 1.61 (0.83, 3.14) | 1.25 (0.63, 2.48) | |
| Q3 | 1.31 (0.66, 2.61) | 1.17 (0.65, 2.12) | 1.03 (0.57, 1.85) | 1.89 (1.02, 3.48) | 1.88 (0.98, 3.58) | |
| Q4 | 1.35 (0.46, 3.95) | 1.03 (0.52, 2.03) | 0.95 (0.50, 1.81) | 1.94 (0.96, 3.94) | 1.77 (0.88, 3.58) | |
| P-trend | 0.64 | 0.87 | 0.87 | 0.063 | 0.075 | |
All models are adjusted for age (y), height (cm), parity (0, 1, 2, 3, 4+), prepregnancy BMI (kg/m2), race (non-Hispanic white, non-Hispanic black, Hispanic, Asian/Pacific Islander), marital status (not married, married, or living with partner), education (<high school, high school or equivalent, some college or associates degree, undergraduate degree, postgraduate degree), income (<30 k, 30 to <40 k, 40 to <50 k, 50 to <75 k, 75 to <100 k, ≥100 k), current job/student status (yes, no), insurance coverage (private/managed care, other), study site (Columbia University, New York Hospital - Queens, Christiana Care Health System, Saint Peter's University Hospital, Medical University of South Carolina, University of Alabama, Northwestern University, Long Beach Memorial Medical Center, University of California - Irvine, Fountain Valley Hospital, Women and Infants Hospital of Rhode Island, Tufts University), infant sex (female, male), total weekly physical activity [metabolic equivalent of task (MET) h/wk], and total daily energy intake (kcal/d). All outcomes, other than birthweight, were also adjusted for measurement date. *P values that remain significant after adjustment for multiple comparisons using the false discovery rate (FDR). AHEI-2010, Alternative Healthy Eating Index-2010; aMED, alternative Mediterranean diet score; DASH, Dietary Approaches to Stop Hypertension; LBW, low birthweight; LGA, large-for-gestational age; NICHD, National Institute of Child Health and Human Development; PCA, principal components analysis; Q, quartile; SGA, small-for-gestational age.
β estimates from linear regression.
ORs from logistic regression.
TABLE 3.
Associations of maternal diet patterns with neonatal anthropometry and preterm birth, NICHD Fetal Growth Studies-Singletons, 2009–20131
| PCA-derived patterns | A priori patterns | |||||
|---|---|---|---|---|---|---|
| β2 (95% CI) | β2 (95% CI) | |||||
| Continuous outcomes | PCA pattern 1 | PCA pattern 2 | AHEI-2010 | aMED | DASH | |
| Length, cm | Q1 | Referent | Referent | Referent | Referent | Referent |
| Q2 | −0.34 (−0.70, 0.02) | −0.17 (−0.53, 0.20) | 0.20 (−0.17, 0.58) | 0.02 (−0.38, 0.42) | 0.11 (−0.22, 0.45) | |
| Q3 | −0.14 (−0.55, 0.29) | 0.12 (−0.31, 0.55) | 0.10 (−0.32, 0.51) | 0.45 (0.08, 0.83) | 0.36 (0.03, 0.69) | |
| Q4 | −0.43 (−1.09, 0.24) | 0.27 (−0.18, 0.71) | 0.51 (0.09, 0.92) | 0.54 (0.10, 0.99) | 0.62 (0.25, 0.99) | |
| P-trend | 0.30 | 0.10 | 0.15 | 0.0059* | 0.0057* | |
| Upper arm length, cm | Q1 | Referent | Referent | Referent | Referent | Referent |
| Q2 | 0.10 (−0.05, 0.25) | 0.05 (−0.10, 0.21) | 0.05 (−0.10, 0.19) | 0.12 (−0.05, 0.30) | 0.10 (−0.06, 0.26) | |
| Q3 | 0.07 (−0.11, 0.24) | 0.07 (−0.10, 0.24) | 0.04 (−0.10, 0.19) | 0.09 (−0.06, 0.24) | 0.25 (0.09, 0.41) | |
| Q4 | 0.14 (−0.12, 0.40) | 0.01 (−0.17, 0.18) | 0.14 (−0.02, 0.29) | 0.25 (0.06, 0.44) | 0.23 (0.06, 0.40) | |
| P-trend | 0.36 | 0.88 | 0.22 | 0.019* | 0.0032* | |
| Upper thigh length, cm | Q1 | Referent | Referent | Referent | Referent | Referent |
| Q2 | 0.01 (−0.18, 0.19) | 0.04 (−0.14, 0.22) | −0.01 (−0.20, 0.17) | 0.02 (−0.18, 0.23) | −0.09 (−0.26, 0.08) | |
| Q3 | −0.09 (−0.29, 0.12) | 0.08 (−0.11, 0.27) | 0.05 (−0.15, 0.24) | 0.08 (−0.11, 0.27) | 0.08 (−0.08, 0.25) | |
| Q4 | −0.11 (−0.43, 0.20) | 0.11 (−0.10, 0.31) | 0.05 (−0.15, 0.26) | 0.13 (−0.10, 0.36) | 0.04 (−0.14, 0.23) | |
| P-trend | 0.43 | 0.34 | 0.56 | 0.23 | 0.80 | |
| Head circumference, cm | Q1 | Referent | Referent | Referent | Referent | Referent |
| Q2 | −0.10 (−0.33, 0.14) | −0.06 (−0.30, 0.18) | 0.14 (−0.09, 0.36) | −0.06 (−0.31, 0.18) | −0.02 (−0.25, 0.21) | |
| Q3 | −0.22 (−0.47, 0.04) | 0.08 (−0.17, 0.33) | 0.09 (−0.15, 0.33) | 0.14 (−0.09, 0.36) | 0.14 (−0.09, 0.37) | |
| Q4 | −0.25 (−0.61, 0.12) | 0.12 (−0.14, 0.38) | 0.26 (0.01, 0.49) | 0.20 (−0.08, 0.48) | 0.26 (−0.004, 0.53) | |
| P-trend | 0.17 | 0.23 | 0.15 | 0.0854 | 0.030 | |
| Abdominal circumference, cm | Q1 | Referent | Referent | Referent | Referent | Referent |
| Q2 | −0.45 (−0.85, −0.05) | −0.16 (−0.55, 0.23) | 0.18 (−0.20, 0.56) | −0.02 (−0.47, 0.43) | −0.08 (−0.47, 0.31) | |
| Q3 | −0.42 (−0.86, 0.02) | 0.06 (−0.39, 0.50) | 0.20 (−0.19, 0.60) | 0.05 (−0.34, 0.45) | 0.07 (−0.31, 0.45) | |
| Q4 | −0.40 (−1.04, 0.24) | 0.04 (−0.42, 0.50) | 0.31 (−0.15, 0.77) | 0.27 (−0.27, 0.80) | 0.28 (−0.20, 0.76) | |
| P-trend | 0.30 | 0.64 | 0.20 | 0.30 | 0.21 | |
| Sum of skinfold, mm | Q1 | Referent | Referent | Referent | Referent | Referent |
| Q2 | −0.43 (−1.15, 0.29) | −0.32 (−1.04, 0.40) | 0.25 (−0.42, 0.91) | −0.40 (−1.11, 0.31) | 0.18 (−0.52, 0.89) | |
| Q3 | −0.73 (−1.53, 0.08) | −0.02 (−0.82, 0.78) | 0.23 (−0.53, 0.99) | 0.15 (−0.50, 0.80) | 0.20 (−0.51, 0.91) | |
| Q4 | −0.93 (−2.14, 0.28) | 0.05 (−0.81, 0.91) | 0.51 (−0.27, 1.29) | 0.67 (−0.12, 1.46) | 0.35 (−0.48, 1.18) | |
| P-trend | 0.13 | 0.68 | 0.32 | 0.0498 | 0.42 | |
| OR3 (95% CI) | OR3 (95% CI) | |||||
| Categorical outcomes, % | PCA pattern 1 | PCA pattern 2 | AHEI-2010 | aMED | DASH | |
| Preterm birth, 6.2% | Q1 | Referent | Referent | Referent | Referent | Referent |
| Q2 | 1.31 (0.69, 2.51) | 0.95 (0.52, 1.74) | 0.96 (0.52, 1.80) | 0.69 (0.37, 1.28) | 0.67 (0.36, 1.24) | |
| Q3 | 1.57 (0.75, 3.27) | 0.87 (0.44, 1.72) | 0.92 (0.48, 1.75) | 0.61 (0.35, 1.05) | 0.76 (0.44, 1.33) | |
| Q4 | 1.60 (0.55, 4.65) | 0.76 (0.35, 1.61) | 0.66 (0.32, 1.36) | 0.49 (0.23, 1.05) | 0.52 (0.26, 1.05) | |
| P-trend | 0.41 | 0.44 | 0.48 | 0.058 | 0.093 | |
All models are adjusted for age (y), height (cm), parity (0, 1, 2, 3, 4+), prepregnancy BMI (kg/m2), race (non-Hispanic white, non-Hispanic black, Hispanic, Asian/Pacific Islander), marital status (not married, married, or living with partner), education (<high school, high school or equivalent, some college or associates degree, undergraduate degree, postgraduate degree), income (<30 k, 30 to <40 k, 40 to <50 k, 50 to <75 k, 75 to <100 k, ≥100 k), current job/student status (yes, no), insurance coverage (private/managed care, other), study site (Columbia University, New York Hospital - Queens, Christiana Care Health System, Saint Peter's University Hospital, Medical University of South Carolina, University of Alabama, Northwestern University, Long Beach Memorial Medical Center, University of California - Irvine, Fountain Valley Hospital, Women and Infants Hospital of Rhode Island, Tufts University), infant sex (female, male), total weekly physical activity [metabolic equivalent of task (MET) h/wk], and total daily energy intake (kcal/d). *P values that remain significant after adjustment for multiple comparisons using the false discovery rate (FDR). AHEI-2010, Alternative Healthy Eating Index-2010; aMED, alternative Mediterranean diet score; DASH, Dietary Approaches to Stop Hypertension; NICHD, National Institute of Child Health and Human Development; PCA, principal components analysis; Q, Quartile.
β estimates from linear regression.
ORs from logistic regression.
Associations of diet patterns with neonatal outcomes after excluding women with obesity who reported smoking are reported in Supplemental Tables 4 and 5. Although some statistically significant results are attenuated, the pattern of associations remained largely the same.
Discussion
In a multiethnic low-risk cohort of pregnant women in the USA, healthy dietary patterns early in pregnancy were positively associated with neonatal size. Specifically, the AHEI-2010, aMed, and DASH dietary patterns, which are known for their cardioprotective effects outside of pregnancy, exhibited significant positive associations with measures such as birthweight, length, upper arm length, sum of skinfold thickness, and head circumference. Adherence to the aMed diet was associated with reduced risk of LBW. However, certain quartiles of the aMed and AHEI-2010 were also positively associated with LGA and macrosomia, suggesting that diet quality is associated with a shift towards larger neonates in the entire birth size distribution. On the other hand, the 2 PCA-derived patterns resembling unhealthy/high-energy diets and healthier diets, were not significantly associated with most anthropometric measures. In the absence of generally accepted pregnancy-specific diet quality scores, these results provide evidence for an association between maternal diet patterns and neonatal outcomes.
Previous studies evaluating the relation between maternal diet patterns (both a priori and data-driven) and neonatal outcomes have been summarized in a recent systematic review and meta-analysis by Chia et al. (14). Pooling 6 studies that used both data-derived and a priori healthy diet patterns (18, 41–45), Chia et al. found that healthier diets were associated with a trend toward a lower risk of SGA (pooled OR: 0.86; 95% CI: 0.73, 1.01). In accordance with these findings, in our study the aMed score was significantly associated with a reduced risk of low birthweight. However, we also observed significant associations of greater aMed and AHEI-2010 scores in some quartiles with increased odds of LGA and macrosomia though the P-trends were not significant. Consistent with this direction of association, a recent study found that poor periconceptional diet quality (per the Healthy Eating Index-2010) was associated with not only a higher risk of SGA and LBW, but also a lower risk of macrosomia (46). Though more studies replicating these findings are needed, adherence to these scores appear to shift neonatal size across the spectrum, and not necessarily result only in beneficial outcomes such as reduction of low birthweight.
Our results expand on the evidence in the recent systematic review and meta-analysis by Chia et al. by adding continuous measures of neonatal anthropometry other than birthweight. We found that the aMed score was positively associated not only with measures that reflect both fat- and fat-free mass (such as birthweight, length, and upper arm length), but also with sum of skinfold thickness which has more discriminate power in capturing total neonatal adiposity (47). Taken together, this may suggest that this dietary pattern has a positive association with overall neonatal size and not with disproportionate changes in body size.
PCA-derived diet patterns similar to those identified in our study have previously been reported in other research about both pregnant (48–51) and nonpregnant populations (52–54). The variance explained by the patterns that we derived also resembles what has been found in previous studies of pregnant women (48, 55, 56). In the Chia et al. review, 3 studies found an association of data-derived unhealthy diet patterns, characterized by high intakes of refined grains, processed meat, and foods high in saturated fat or sugar, with lower birthweight (26, 57, 58). However, associations of PCA pattern 1 with birthweight and most anthropometric measures in our data were null. Of note, PCA pattern 2 had a moderate agreement with aMed (weighted κ = 0.413). However, the aMed index includes components like legumes and nuts that do not characterize (i.e., load highly on) PCA pattern 2. This distinction hints that even the “health-conscious or prudent” diet pattern identified in our data has room for improvement in diet quality and may, in part, explain the null findings of PCA pattern 2 compared with the significant associations observed with aMed.
Healthy diet patterns may positively influence favorable fetal growth by providing a balanced supply of essential nutrients. Another possible mechanism could be through reduction of the inflammatory pathway that increases risk of hypertensive disorders of pregnancy and consequently the risk of fetal growth restriction (59–61). High concentrations of circulating inflammatory and oxidative stress markers have also been associated with unfavorable reductions in neonatal size (62–64) and healthier diets have been associated with lower oxidative stress and inflammation during pregnancy (65). On the other hand, the pathways through which healthier diets may be associated with LGA and macrosomia are unknown. Pesticide residue exposure from fruits and vegetables has been hypothesized to disrupt glucose metabolism (66), but associations with gestational diabetes (67–69) and fetal growth (70, 71) have yielded mixed findings. ω-3 long-chain PUFAs commonly found in fish (a component of diet quality scores) have also been associated with larger head circumference (72), but this was in the context of supplementation trials and the effect size was small. Therefore, our findings require replication and better mechanistic insight.
A fundamental strength of our study was that we used a large, multiethnic, and well-characterized cohort of pregnant women from a substantial number of sites across the USA. Our analysis examined multiple neonatal anthropometric measurements, which broadens the insights beyond the approach of most previous studies that focus on birthweight alone as the outcome measure. Likewise, we employed multiple measures of diet patterns, including 1 derived from the data and 3 a priori indices. We consider the addition of a data-driven method to be useful and complementary in this analysis because the a priori scores were developed based on associations with chronic diseases and not pregnancy-related outcomes. Though we accounted for several indicators of socioeconomic status, residual confounding, especially by macrolevel factors associated with diet (73, 74) and pregnancy outcomes (75, 76) may be present. The cohort reflected criteria for selection that screened women based on the absence of chronic disease. These restrictions minimize the generalizability of our results to the entire population of pregnant women in the USA. Nonetheless, examining the relation of diet patterns with neonatal outcomes in this low-risk population could be beneficial by minimizing the chances that certain unmeasured risk factors may confound the association. Another limitation of our analysis was that diet was evaluated at a single point in time, which may fail to account for potential changes in diet patterns as pregnancy progresses. Multiple studies, however, reported that maternal diets tend to change minimally from preconception throughout the duration of pregnancy, and diet patterns identified in early pregnancy are likely to persist for the remainder of the pregnancy (77, 78).
In conclusion, in this cohort of mostly low-risk pregnant women, we found evidence that pre- and early pregnancy healthful diet quality indices, especially the aMed score, were associated with larger neonates across the entire birthweight distribution. Further work is needed to understand the biological mechanisms involved. Most existing knowledge about the healthiness of diets is derived from studies in nonpregnant populations. Our research reinforces the value of identifying maternal diet patterns and developing diet indices that are predictive of optimal fetal growth, which should be areas of active research in the future.
Supplementary Material
Acknowledgements
The clinical centers involved in data collection for the NICHD Fetal Growth Studies are (in alphabetical order): Christina Care Health Systems; Columbia University; Fountain Valley Hospital (California); Long Beach Memorial Medical Center; New York Hospital (Queens), Northwestern University; University of Alabama at Birmingham; University of California, Irvine, Medical University of South Carolina; Saint Peter's University Hospital; Tufts University; and Women and Infants Hospital of Rhode Island. C-TASC and The EMMES Corporation were the data coordinating centers that provided data and imaging support for this multisite study.
The authors’ responsibilities were as follows—JG, CZ, KLG, SNH: data acquisition; SFY, SLM, JG, SNH: study design; SFY, ML: data analysis; SFY: wrote the first draft of the manuscript; and all authors: contributed to critical discussion, revisions to the manuscript, and read and approved the final manuscript. The authors report no conflicts of interest.
Notes
This research was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development intramural funding as well as the American Recovery and Reinvestment Act funding (contract numbers HHSN275200800013C, HHSN275200800002I, HHSN27500006, HHSN275200800003IC, HHSN275200800014C, HHSN275200800012C, HHSN275200800028C, HHSN275201000009C, and HHSN275201000001Z).
Supplemental Figure 1 and Supplemental Tables 1–5 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: AHEI, Alternate Healthy Eating Index; aMed, alternate Mediterranean diet; DASH, Dietary Approaches to Stop Hypertension; FDR, false discovery rate; LBW, low birthweight; LGA, large-for-gestational age; NICHD, National Institute of Child Health and Human Development; PCA, principal components analysis; SGA, small-for-gestational age.
Contributor Information
Samrawit F Yisahak, Office of the Director, Division of Intramural Population Health Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD, USA.
Sunni L Mumford, Epidemiology Branch, Division of Intramural Population Health Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD, USA.
Jagteshwar Grewal, Office of the Director, Division of Intramural Population Health Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD, USA.
Mengying Li, Epidemiology Branch, Division of Intramural Population Health Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD, USA.
Cuilin Zhang, Epidemiology Branch, Division of Intramural Population Health Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD, USA.
Katherine L Grantz, Epidemiology Branch, Division of Intramural Population Health Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD, USA.
Stefanie N Hinkle, Epidemiology Branch, Division of Intramural Population Health Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD, USA.
Data Availability
The data and codebook, along with a set of guidelines for researchers applying for the data, will be posted in the future to a data-sharing site, the Eunice Kennedy Shriver National Institute of Child Health and Human Development/Division of Intramural Population Health Research Biospecimen Repository Access and Data Sharing (https://brads.nichd.nih.gov) (BRADS). The analytic code for this manuscript is available upon request.
References
- 1. Chauhan SP, Rice MM, Grobman WA, Bailit J, Reddy UM, Wapner RJ, Varner MW, Thorp Jr JM, Leveno KJ, Caritis SN. Neonatal morbidity of small- and large-for-gestational-age neonates born at term in uncomplicated pregnancies. Obstetrics & Gynecology. 2017;130(3):511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Spiegel E, Shoham-Vardi I, Sergienko R, Landau D, Sheiner E. The association between birth weight at term and long-term endocrine morbidity of the offspring. J Matern Fetal Neonatal Med. 2019;32(16):2657–61. [DOI] [PubMed] [Google Scholar]
- 3. Oken E, Kleinman KP, Olsen SF, Rich-Edwards JW, Gillman MW. Associations of seafood and elongated n-3 fatty acid intake with fetal growth and length of gestation: results from a US pregnancy cohort. Am J Epidemiol. 2004;160(8):774–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lagiou P, Mucci L, Tamimi R, Kuper H, Lagiou A, Hsieh C-C, Trichopoulos D. Micronutrient intake during pregnancy in relation to birth size. Eur J Nutr. 2005;44(1):52–9. [DOI] [PubMed] [Google Scholar]
- 5. Moeller SM, Reedy J, Millen AE, Dixon LB, Newby PK, Tucker KL, Krebs-Smith SM, Guenther PM. Dietary patterns: challenges and opportunities in dietary patterns research: an Experimental Biology workshop, April 1, 2006. J Am Diet Assoc. 2007;107(7):1233–9. [DOI] [PubMed] [Google Scholar]
- 6. Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3–9. [DOI] [PubMed] [Google Scholar]
- 7. Jacques PF, Tucker KL. Are dietary patterns useful for understanding the role of diet in chronic disease? Am J Clin Nutr. 2001;73(1):1–2. [DOI] [PubMed] [Google Scholar]
- 8. Philipps C, Johnson NE. The impact of quality of diet and other factors on birth weight of infants. Am J Clin Nutr. 1977;30(2):215–25. [DOI] [PubMed] [Google Scholar]
- 9. Bodnar LM, Siega-Riz AM. A Diet Quality Index for pregnancy detects variation in diet and differences by sociodemographic factors. Public Health Nutr. 2002;5(6):801–9. [DOI] [PubMed] [Google Scholar]
- 10. Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fung TT, McCullough ML, Newby P, Manson JE, Meigs JB, Rifai N, Willett WC, Hu FB. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2005;82(1):163–73. [DOI] [PubMed] [Google Scholar]
- 12. Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, Simons-Morton DG. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344(1):3–10. [DOI] [PubMed] [Google Scholar]
- 13. Harmon BE, Boushey CJ, Shvetsov YB, Ettienne R, Reedy J, Wilkens LR, Le Marchand L, Henderson BE, Kolonel LN. Associations of key diet-quality indexes with mortality in the Multiethnic Cohort: the Dietary Patterns Methods Project. Am J Clin Nutr. 2015;101(3):587–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chia AR, Chen LW, Lai JS, Wong CH, Neelakantan N, van Dam RM, Chong MF. Maternal dietary patterns and birth outcomes: a systematic review and meta-analysis. Advances in Nutrition (Bethesda, Md). 2019;10(4):685–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park S-Y, Murphy SP, Wilkens LR, Yamamoto JF, Sharma S, Hankin JH, Henderson BE, Kolonel LN. Dietary patterns using the food guide pyramid groups are associated with sociodemographic and lifestyle factors: The Multiethnic Cohort Study. J Nutr. 2005;135(4):843–9. [DOI] [PubMed] [Google Scholar]
- 16. Sharma S, Wilkens LR, Shen L, Kolonel LN. Dietary sources of five nutrients in ethnic groups represented in the Multiethnic Cohort. Br J Nutr. 2013;109(8):1479–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shapiro AL, Kaar JL, Crume TL, Starling AP, Siega-Riz AM, Ringham BM, Glueck DH, Norris JM, Barbour LA, Friedman J. Maternal diet quality in pregnancy and neonatal adiposity: the Healthy Start Study. Int J Obes. 2016;40(7):1056–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martin CL, Sotres-Alvarez D, Siega-Riz AM. Maternal dietary patterns during the second trimester are associated with preterm birth. J Nutr. 2015;145(8):1857–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miller V, Webb P, Micha R, Mozaffarian D. Defining diet quality: a synthesis of dietary quality metrics and their validity for the double burden of malnutrition. The Lancet Planetary Health. 2020;4(8):e352–e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Louis GMB, Grewal J, Albert PS, Sciscione A, Wing DA, Grobman WA, Newman RB, Wapner R, D'Alton ME, Skupski Det al. Racial/ethnic standards for fetal growth: the NICHD Fetal Growth Studies. Am J Obstet Gynecol. 2015;213(4):449. e1-449.e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grewal J, Grantz KL, Zhang C, Sciscione A, Wing DA, Grobman WA, Newman RB, Wapner R, D'Alton ME, Skupski D. Cohort Profile: NICHD fetal growth studies – singletons and twins. Int J Epidemiol. 2018;47(1):25–l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Radesky JS, Oken E, Rifas-Shiman SL, Kleinman KP, Rich-Edwards JW, Gillman MW. Diet during early pregnancy and development of gestational diabetes. Paediatr Perinat Epidemiol. 2007;22(1):47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348(26):2599–608. [DOI] [PubMed] [Google Scholar]
- 24. Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168(7):713–20. [DOI] [PubMed] [Google Scholar]
- 25. Bowman SA, Friday JE, Moshfegh AJ. My Pyramid Equivalents Database, 2.0 for USDA survey foods, 2003–2004: documentation and user guide. US Department of Agriculture;2008. [Google Scholar]
- 26. Northstone K, Ness A, Emmett P, Rogers I. Adjusting for energy intake in dietary pattern investigations using principal components analysis. Eur J Clin Nutr. 2008;62(7):931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chasan-Taber L, Schmidt MD, Roberts DE, Hosmer D, Markenson G, Freedson PS. Development and validation of a pregnancy physical activity questionnaire. Med Sci Sports Exerc. 2004;36(10):1750–60. [DOI] [PubMed] [Google Scholar]
- 28. Pugh SJ, Albert PS, Kim S, Grobman W, Hinkle SN, Newman RB, Wing DA, Grantz KL. Patterns of gestational weight gain and birthweight outcomes in the Eunice Kennedy Shriver National Institute of Child Health and Human Development fetal growth studies-singletons: a prospective study. Am J Obstet Gynecol. 2017;217(3):346.e1.e1-.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. National Health and Nutritional Examination Survey. Anthropometry Procedures Manual. 2007–2008. [Internet]. Available from: https://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/manual_an.pdf [accessed March 2019]. [Google Scholar]
- 30. Catalano PM, Thomas AJ, Avallone DA, Amini SB. Anthropometric estimation of neonatal body composition. Am J Obstet Gynecol. 1995;173(4):1176–81. [DOI] [PubMed] [Google Scholar]
- 31. Fok T, Hon K, Wong E, Ng P, So H, Lau J, Chow C, Lee W, Group H. Trunk anthropometry of Hong Kong Chinese infants. Early Hum Dev. 2005;81(9):781–90. [DOI] [PubMed] [Google Scholar]
- 32. Rodríguez G, Samper MP, Ventura P, Perez-Gonzalez JM. Sex-specific charts for abdominal circumference in term and near-term Caucasian newborns. J Perinat Med. 2008;36(6):527–30. [DOI] [PubMed] [Google Scholar]
- 33. Duryea EL, Hawkins JS, McIntire DD, Casey BM, Leveno KJ. A revised birth weight reference for the United States. Obstet Gynecol. 2014;124(1):16–22. [DOI] [PubMed] [Google Scholar]
- 34. Hu FB, Rimm E, Smith-Warner SA, Feskanich D, Stampfer MJ, Ascherio A, Sampson L, Willett WC. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr. 1999;69(2):243–9. [DOI] [PubMed] [Google Scholar]
- 35. Millen BA, Quatromoni PA, Copenhafer DL, Demissie S, O'Horo CE, D'Agostino RB. Validation of a dietary pattern approach for evaluating nutritional risk: the Framingham Nutrition Studies. J Am Diet Assoc. 2001;101(2):187–94. [DOI] [PubMed] [Google Scholar]
- 36. Quatromoni PA, Copenhafer DL, Demissie S, D'agostino RBD, O'horo CE, Nam B-H, Millen BE. The internal validity of a dietary pattern analysis. The Framingham Nutrition Studies. J Epidemiol Community Health. 2002;56(5):381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ford N, Jaacks L, Martorell R, Mehta KN, Perrine C, Ramirez-Zea M, Stein A. Dietary patterns and cardio-metabolic risk in a population of Guatemalan young adults. BMC Nutrition. 2017;3(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ananth CV, Schisterman EF. Confounding, causality, and confusion: the role of intermediate variables in interpreting observational studies in obstetrics. Am J Obstet Gynecol. 2017;217(2):167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhou XH, Eckert GJ, Tierney WM. Multiple imputation in public health research. Statist Med. 2001;20(9–10):1541–9. [DOI] [PubMed] [Google Scholar]
- 40. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74. [PubMed] [Google Scholar]
- 41. Gresham E, Collins CE, Mishra GD, Byles JE, Hure AJ. Diet quality before or during pregnancy and the relationship with pregnancy and birth outcomes: the Australian Longitudinal Study on Women's Health. Public Health Nutr. 2016;19(16):2975–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mikkelsen TB, Osterdal ML, Knudsen VK, Haugen M, Meltzer HM, Bakketeig L, Olsen SF. Association between a Mediterranean-type diet and risk of preterm birth among Danish women: a prospective cohort study. Acta Obstet Gynecol Scand. 2008;87(3):325–30. [DOI] [PubMed] [Google Scholar]
- 43. Chia A-R, Tint M-T, Han CY, Chen L-W, Colega M, Aris IM, Chua M-C, Tan K-H, Yap F, Shek LP-C. Adherence to a healthy eating index for pregnant women is associated with lower neonatal adiposity in a multiethnic Asian cohort: the Growing Up in Singapore Towards healthy Outcomes (GUSTO) Study. Am J Clin Nutr. 2018;107(1):71–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hillesund ER, Øverby NC, Engel SM, Klungsøyr K, Harmon QE, Haugen M, Bere E. Associations of adherence to the New Nordic Diet with risk of preeclampsia and preterm delivery in the Norwegian Mother and Child Cohort Study (MoBa). Eur J Epidemiol. 2014;29(10):753–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Saunders L, Guldner L, Costet N, Kadhel P, Rouget F, Monfort C, Thomé JP, Multigner L, Cordier S. Effect of a Mediterranean diet during pregnancy on fetal growth and preterm delivery: results from a French Caribbean Mother–Child Cohort Study (TIMOUN). Paediatr Perinat Epidemiol. 2014;28(3):235–44. [DOI] [PubMed] [Google Scholar]
- 46. Yee LM, Silver RM, Haas DM, Parry S, Mercer BM, Iams J, Wing D, Parker CB, Reddy UM, Wapner RJ. Quality of periconceptional dietary intake and maternal and neonatal outcomes. Am J Obstet Gynecol. 2020;223(1):121.e1–121.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen L-W, Tint M-T, Fortier MV, Aris IM, Shek LP, Tan KH, Chan S-Y, Gluckman PD, Chong Y-S, Godfrey KM. Which anthropometric measures best reflect neonatal adiposity? Int J Obes. 2018;42(3):501–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Crozier SR, Inskip HM, Godfrey KM, Robinson SM. Dietary patterns in pregnant women: a comparison of food-frequency questionnaires and 4 d prospective diaries. Br J Nutr. 2008;99(4):869–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lange NE, Rifas-Shiman SL, Camargo Jr CA, Gold DR, Gillman MW, Litonjua AA. Maternal dietary pattern during pregnancy is not associated with recurrent wheeze in children. J Allergy Clin Immunol. 2010;126(2):250–5.. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Knudsen VK, Orozova-Bekkevold IM, Mikkelsen TB, Wolff S, Olsen SF. Major dietary patterns in pregnancy and fetal growth. Eur J Clin Nutr. 2008;62:463. doi: 10.1038/sj.ejcn.1602745. [DOI] [PubMed] [Google Scholar]
- 51. Chen X, Zhao D, Mao X, Xia Y, Baker P, Zhang H. Maternal dietary patterns and pregnancy outcome. Nutrients. 2016;8(6):351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hu FB, Rimm EB, Stampfer MJ, Ascherio A, Spiegelman D, Willett WC. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am J Clin Nutr. 2000;72(4):912–21. [DOI] [PubMed] [Google Scholar]
- 53. Crozier SR, Robinson SM, Borland SE, Inskip HM, Group S. Dietary patterns in the Southampton Women's Survey. Eur J Clin Nutr. 2006;60(12):1391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Newby P, Tucker KL. Empirically derived eating patterns using factor or cluster analysis: a review. Nutr Rev. 2004;62(5):177–203. [DOI] [PubMed] [Google Scholar]
- 55. Bouwland-Both MI, Steegers-Theunissen RP, Vujkovic M, Lesaffre EM, Mook-Kanamori DO, Hofman A, Lindemans J, Russcher H, Jaddoe VW, Steegers EA. A periconceptional energy-rich dietary pattern is associated with early fetal growth: the Generation R study. BJOG. 2013;120(4):435–45. [DOI] [PubMed] [Google Scholar]
- 56. Northstone K, Emmett PM, Rogers I. Dietary patterns in pregnancy and associations with nutrient intakes. Br J Nutr. 2008;99(2):406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wolff CB, Wolff HK. Maternal eating patterns and birth weight of Mexican American infants. Nutr Health. 1995;10(2):121–34. [DOI] [PubMed] [Google Scholar]
- 58. Coelho Nde L, Cunha DB, Esteves AP, Lacerda EM, Theme Filha MM. Dietary patterns in pregnancy and birth weight. Rev Saúde Públ. 2015;49:62. doi: 10.1590/s0034-8910.2015049005403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Brantsæter AL, Haugen M, Samuelsen SO, Torjusen H, Trogstad L, Alexander J, Magnus P, Meltzer HM. A dietary pattern characterized by high intake of vegetables, fruits, and vegetable oils is associated with reduced risk of preeclampsia in nulliparous pregnant Norwegian women. J Nutr. 2009;139(6):1162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Clausen T, Slott M, Solvoll K, Drevon CA, Vollset SE, Henriksen T. High intake of energy, sucrose, and polyunsaturated fatty acids is associated with increased risk of preeclampsia. Am J Obstet Gynecol. 2001;185(2):451–8. [DOI] [PubMed] [Google Scholar]
- 61. Ødegård RA, Vatten LJ, Nilsen ST, Salvesen KÅ, Austgulen R. Preeclampsia and fetal growth. Obstet Gynecol. 2000;96(6):950–5. [PubMed] [Google Scholar]
- 62. Biri A, Bozkurt N, Turp A, Kavutcu M, Himmetoglu Ö, Durak I. Role of oxidative stress in intrauterine growth restriction. Gynecol Obstet Invest. 2007;64(4):187–92. [DOI] [PubMed] [Google Scholar]
- 63. Potdar N, Singh R, Mistry V, Evans MD, Farmer PB, Konje JC, Cooke MS. First-trimester increase in oxidative stress and risk of small-for-gestational-age fetus. BJOG. 2009;116(5):637–42. [DOI] [PubMed] [Google Scholar]
- 64. Englund-Ögge L, Brantsæter AL, Sengpiel V, Haugen M, Birgisdottir BE, Myhre R, Meltzer HM, Jacobsson B. Maternal dietary patterns and preterm delivery: results from large prospective cohort study. BMJ. 2014;348:g1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kim H, Hwang J, Ha E, Park H, Ha M, Lee S, Hong Y-C, Chang N. Fruit and vegetable intake influences the association between exposure to polycyclic aromatic hydrocarbons and a marker of oxidative stress in pregnant women. Eur J Clin Nutr. 2011;65(10):1118–25. [DOI] [PubMed] [Google Scholar]
- 66. Guo W, Pan B, Sakkiah S, Yavas G, Ge W, Zou W, Tong W, Hong H. Persistent organic pollutants in food: contamination sources, health effects and detection methods. IJERPH. 2019;16(22):4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Saldana TM, Basso O, Hoppin JA, Baird DD, Knott C, Blair A, Alavanja MC, Sandler DP. Pesticide exposure and self-reported gestational diabetes mellitus in the Agricultural Health Study. Diabetes Care. 2007;30(3):529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Saunders L, Kadhel P, Costet N, Rouget F, Monfort C, Thomé J-P, Guldner L, Cordier S, Multigner L. Hypertensive disorders of pregnancy and gestational diabetes mellitus among French Caribbean women chronically exposed to chlordecone. Environ Int. 2014;68:171–6. [DOI] [PubMed] [Google Scholar]
- 69. Shapiro GD, Dodds L, Arbuckle TE, Ashley-Martin J, Ettinger AS, Fisher M, Taback S, Bouchard MF, Monnier P, Dallaire R. Exposure to organophosphorus and organochlorine pesticides, perfluoroalkyl substances, and polychlorinated biphenyls in pregnancy and the association with impaired glucose tolerance and gestational diabetes mellitus: The MIREC Study. Environ Res. 2016;147:71–81. [DOI] [PubMed] [Google Scholar]
- 70. Chiu Y-H, Williams PL, Gillman MW, Hauser R, Rifas-Shiman SL, Bellavia A, Fleisch AF, Oken E, Chavarro JE. Maternal intake of pesticide residues from fruits and vegetables in relation to fetal growth. Environ Int. 2018;119:421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Berkowitz GS, Wetmur JG, Birman-Deych E, Obel J, Lapinski RH, Godbold JH, Holzman IR, Wolff MS. In utero pesticide exposure, maternal paraoxonase activity, and head circumference. Environ Health Perspect. 2004;112(3):388–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Szajewska H, Horvath A, Koletzko B. Effect of n−3 long-chain polyunsaturated fatty acid supplementation of women with low-risk pregnancies on pregnancy outcomes and growth measures at birth: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2006;83(6):1337–44. [DOI] [PubMed] [Google Scholar]
- 73. Singleton CR, Winkler M, Houghtaling B, Adeyemi OS, Roehll AM, Pionke J, Anderson Steeves E. Understanding the intersection of race/ethnicity, socioeconomic status, and geographic location: a scoping review of US consumer food purchasing. IJERPH. 2020;17(20):7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Laraia BA, Siega-Riz AM, Kaufman JS, Jones SJ. Proximity of supermarkets is positively associated with diet quality index for pregnancy. Prev Med. 2004;39(5):869–75. [DOI] [PubMed] [Google Scholar]
- 75. Sealy-Jefferson S, Giurgescu C, Helmkamp L, Misra DP, Osypuk TL. Perceived physical and social residential environment and preterm delivery in African-American women. Am J Epidemiol. 2015;182(6):485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Morenoff JD. Neighborhood mechanisms and the spatial dynamics of birth weight. Am J Sociol. 2003;108(5):976–1017. [DOI] [PubMed] [Google Scholar]
- 77. Crozier SR, Robinson SM, Godfrey KM, Cooper C, Inskip HM. Women's dietary patterns change little from before to during pregnancy. J Nutr. 2009;139(10):1956–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cuco G, Fernandez-Ballart J, Sala J, Viladrich C, Iranzo R, Vila J, Arija V. Dietary patterns and associated lifestyles in preconception, pregnancy and postpartum. Eur J Clin Nutr. 2006;60(3):364–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and codebook, along with a set of guidelines for researchers applying for the data, will be posted in the future to a data-sharing site, the Eunice Kennedy Shriver National Institute of Child Health and Human Development/Division of Intramural Population Health Research Biospecimen Repository Access and Data Sharing (https://brads.nichd.nih.gov) (BRADS). The analytic code for this manuscript is available upon request.