Fig. 1 |. Overview of the neurovascular unit in healthy and tumour-bearing brains.
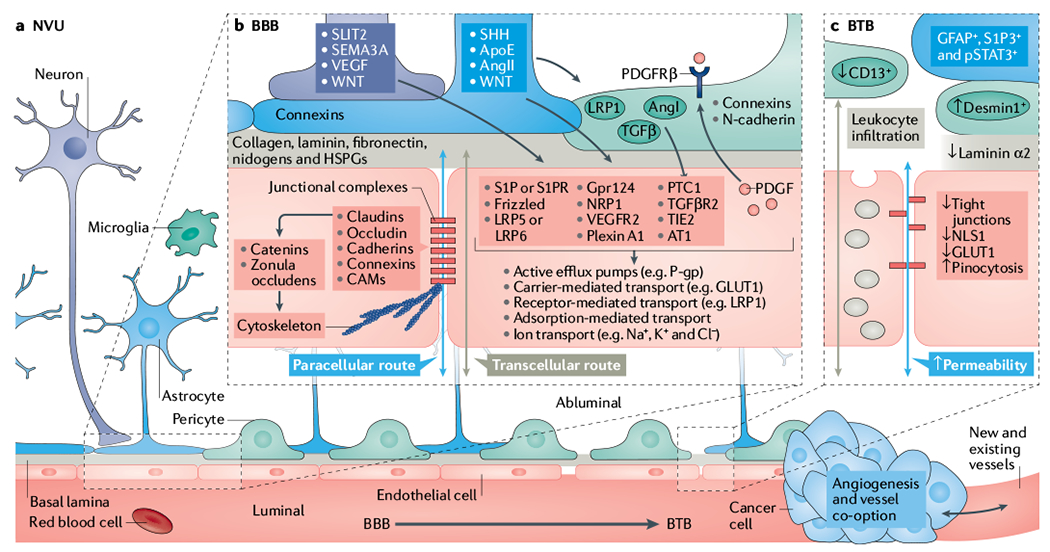
a | Schematic representation of capillaries in the neurovascular unit (NVU) with the intact blood–brain barrier (BBB, bottom left) and the disrupted blood–tumour barrier (BTB, bottom right) in the neuroparenchyma. BBB development and permeability is dictated by signalling and structural mechanisms that are regulated by multiple cells within the NVU. These mechanisms control both paracellular and transcellular routes and, ultimately, vessel permeability in the central nervous system (CNS). b | Schematic representation of notable cellular and molecular components that regulate the development, maturation and function of endothelial cells (ECs) (red) and the NVU (refer to main text for relevant references). Neuronal (purple) and non-neuronal cells regulate the expression of transport and tight junction proteins in ECs, which in turn may ‘loosen’ or ‘tighten’ the BBB. Here, we depict examples of key signalling pathways connecting astrocytes (blue), pericytes (green) and neurons to ECs. Together or individually, these pathways will alter transcellular transport by changing the expression of transporters and the paracellular route by disrupting junctional protein complexes. Of note, ECs reciprocally regulate components of the NVU. For example, EC-secreted transforming growth factor-β (TGFβ) can activate cognate receptor on pericytes. During development and maturation, glial cells, pericytes and neurons regulate EC behaviour via multiple ligands and receptors, which in turn activate downstream signalling cascades (for example, Frizzled, G protein-coupled receptor 124 (GPR124), β-catenin, GLI, PI3K, SRC and the p38 MAPK) that dictate expression of junctional and transcytosis proteins and control CNS homeostasis. For example, astrocytes directly modulate NVU demands such as water content in the neuroparenchymal space via the major water channel protein aquaporin 4 (AQP4), regulate immune cell and cancer cell infiltration via specific chemokine and cytokine production, regulate BBB permeability and integrity in part by angiotensin (AngI and AngII), apolipoprotein E (ApoE) and retinoic acid, and regulate pericyte distribution. c | In the BTB, NVU integrity and endothelial permeability is compromised due to disruption of the NVU, including displacement of astrocytes (blue) and pericytes (green), neurovascular decoupling, altered pericyte populations and changes in EC tight junctions and transcytosis mechanisms. Additional vascular-related phenotypes such as hypoxia, oedema, angiogenesis and tumour-vessel co-option can influence the NVU in brain tumours. Although BBB features remain present during tumour development, in particular at the cancer–neuroparenchyma edge, the BTB displays increased and heterogeneous permeability. Tumour progression leads to BTB structural changes including neuronal death, astrocyte endfeet displacement (from primary and metastatic cancer cells) and heterogenous pericyte and astrocyte subpopulations, all of which can reduce the barrier functions of the CNS endothelium. Intracellular vesicular transport is represented by grey-coloured vesicles in the schematic. AT1, angiotensin II receptor type 1; CAM, cell adhesion molecule; GLUT1, glucose transporter 1; HSPG, heparan sulfate proteoglycan; LRP, low-density lipoprotein receptor-related protein; NLS1, sodium-dependent lysophosphatidylcholine symporter 1; NRP1, neuropilin receptor 1; PDGF, platelet-derived growth factor; PDGFRβ, platelet-derived growth factor receptor β; P-gp, P-glycoprotein; PTC1, protein patched homologue 1; S1P, sphingosine-1-phosphate; S1PR, sphingosine-1-phosphate receptor; SEMA3A, semaphorin 3A; SHH, sonic hedgehog; SLIT2, Slit homologue 2 protein; TGFβR2, transforming growth factor-β receptor 2; TIE2, tyrosine kinase with Ig and EGF homology domains 2; VEGF, vascular endothelial growth factor; VEGFR2, vascular endothelial growth factor receptor 2.
