Fig. 3 |. Improving drug delivery through the BBB/BTB.
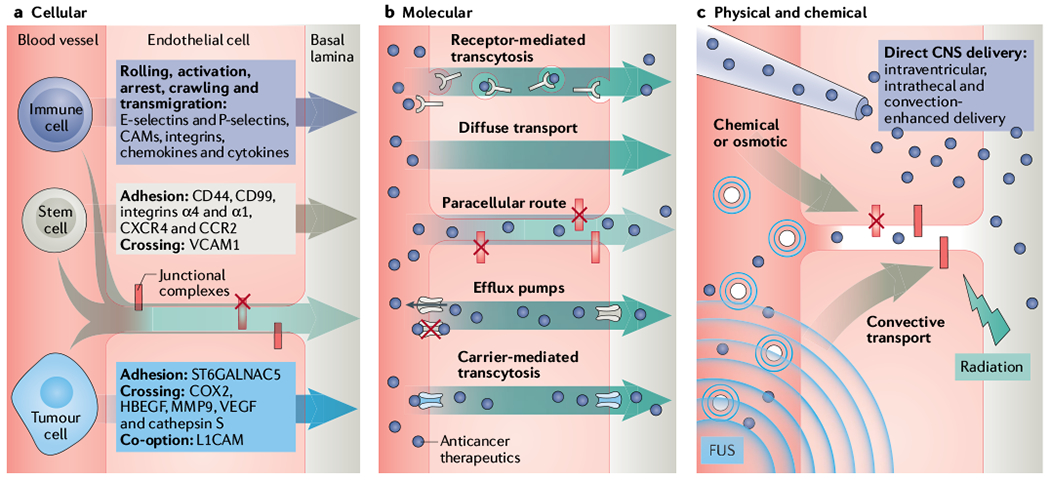
Schematic presentation of key molecular, cellular and physical mechanisms and systems to overcome the blood–brain barrier and blood–tumour barrier (BBB/BTB). a | The BBB prevents cellular extravasation into the neuroparenchyma unless compromised by circulating cells equipped with necessary ‘brain-tropic’ molecular components197, including soluble and membrane-bound proteins, to disturb the BBB integrity. Immune cell extravasation into the central nervous system (CNS) occurs by the following steps: rolling, activation, arrest, crawling, transmigration. The transcellular route is preferred when the BBB is intact, whereas the paracellular route is preferred when there is reduced tight junction integrity (red X = disrupted junction) and formation of intercellular gaps. Transmigration across the BBB is mediated by actin-containing protrusive structures and occurs on the timescale of minutes. Stem cells that have been engineered to contain anticancer cargo localize to sites of neuroinflammation and display coordinated rolling and adhesion behaviour, and transcellular and paracellular transmigration. In contrast to immune cells, mesenchymal stem cell (MSC) transmigration does not involve substantial lateral crawling. Stem cells migrate by the paracellular or transcellular route through discrete gaps or pores in the CNS endothelium. Stem cell transmigration is mediated by membrane blebbing and occurs on the timescale of hours. In contrast, circulating metastatic cancer cells that accumulate in the CNS capillary bed must express specific proteins in order to adhere and breach the BBB. Metastatic cells express proteases that disrupt junctional complexes. Although preclinical studies show that this process may occur within days, the time course of symptomatic brain metastasis in patients varies greatly (from months to years after metastatic dissemination of primary tumour). b | Several molecular strategies are employed to hijack or bypass barriers posed by the neurovascular unit (NVU) (described in main text and TABLE 1). Anticancer therapeutics (purple circles) can be designed with low affinity to efflux pumps or may hijack carriers or receptor-mediated transcytosis mechanisms. Alternatively, inhibitors of efflux pump or junctional complexes can be used to limit the clearance of drugs from the tumours. c | Direct delivery into the neuroparenchyma and physical disruption of the BBB/BTB. Specifically, focused ultrasound (FUS, indicated by the waves) with microbubbles (concentric small blue circles), radiation, osmotic, direct and convective-mediated delivery of therapeutics into the CNS is depicted. CAM, cell adhesion molecule; CCR2, CC-chemokine receptor 2; COX2, cyclooxygenase 2; CXCR4, CXC-chemokine receptor 4; HBECF, proheparin-binding ECF-like growth factor; L1CAM, L1 cell adhesion molecule; ST6GALNAC5, a 2,6-sialyltransferase; MMP9, matrix metalloproteinase 9; VCAM1, vascular cell adhesion protein 1; VECF, vascular endothelial growth factor.
