Figure 2.
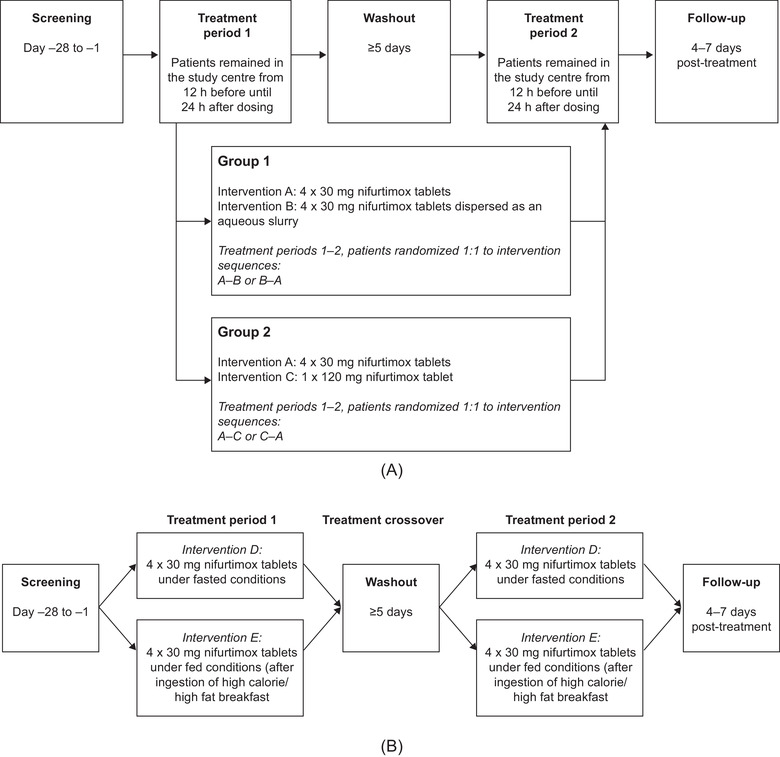
Study designs. (A) In study A, group 1 investigated the pharmacokinetic characteristics of nifurtimox administered as tablets or slurry, and group 2 investigated the bioequivalence of the 30‐ and 120‐mg tablets. Intervention sequences are shown in each group; all dosing was under fed conditions. (B) Study B compared the bioavailability of nifurtimox under fed and fasting conditions.
