Figure 1.
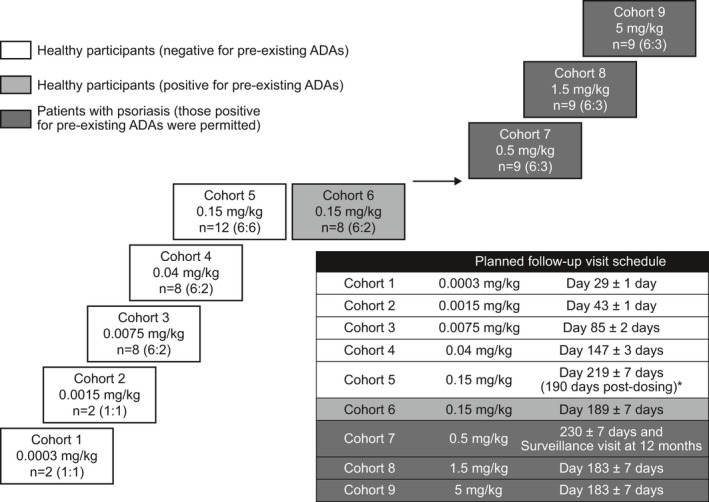
Clinical study design. *Cohort 5 included a pre‐treatment delayed‐type hypersensitivity challenge. Dosing with GSK2831781 occurred on day 29, hence, follow‐up is correspondingly longer relative to study day 1. ADA, anti‐drug antibodies; n, total participants (GSK2831781:placebo).
