Abstract
Elite controllers (ECs) are people living with human immunodeficiency virus (HIV) who spontaneously control viral replication without antiretroviral therapy. We observed that elevated anti-cytomegalovirus (CMV) immunoglobulin G (IgG) levels correlated with annual CD4 T-cell count decay in ECs independently of age, sex, and human leukocyte antigen (HLA) type. Elevated anti-CMV titers may favor disease progression in ECs.
Keywords: cytomegalovirus, HIV, elite controllers, CD4 decay
Current therapeutic guidelines recommend that people living with human immunodeficiency virus (HIV; PLWH) receive antiretroviral therapy (ART) as early as possible to prevent CD4 T-cell decay and inflammation and limit HIV disease progression [1].
Elite controllers (ECs) are a rare subgroup of PLWH who maintain undetectable plasma HIV viral load without ART [2]. Several mechanisms for control of HIV replication have been identified and include protective human leukocyte antigen (HLA) alleles enhancing CD8 T-cell and innate immune responses. However, chronic inflammation, gradual CD4 T-cell decay, and increased incidence of non-AIDS comorbidities such as cardiovascular disease and cancer have been reported in ECs [3, 4]. It is not yet understood which factors are associated with loss of HIV viral control or CD4 T-cell decay when HIV viral replication remains undetectable [5].
We and others have shown that cytomegalovirus (CMV) coinfection contributes to HIV-associated immune activation and inflammation in non-ECs including ART-naive and ART-treated PLWH [6–9]. Notably, we showed that CMV infection and anti-CMV immunoglobulin G (IgG) levels were associated with gut damage and microbial translocation of bacterial lipopolysaccharide (LPS) [9]. Moreover, clinical intervention of asymptomatic CMV infection with the anti-CMV medication valganciclovir for 8 weeks reduced T-cell activation among ART-treated PLWH [10]. Herein, we sought to investigate in ECs whether CMV coinfection is associated with CD4 T-cell decay, a key marker for HIV disease progression.
METHODS
Study Design
This study included 23 adult PLWH from the Canadian Cohort of HIV-infected Slow Progressors (CIHR/CTN 247) [5]. We selected ECs who maintained HIV plasma viremia <1.7 log10 copies/mL and CD4 T-cell count >200 cells/μL who never received ART. Viral load blips <200 copies/mL were allowed.
Clinical Laboratory Measurements
Blood samples were collected from participants in Canada to isolate peripheral blood mononuclear cells and plasma, which were then stored in liquid nitrogen and at −80oC, respectively [5]. Quantification of plasma HIV viral load was done using the RealTime HIV-1 assay (Abbott Laboratories). Absolute CD4 and CD8 T-cell counts were measured by clinical labs using flow cytometry. HLA typing was performed on whole blood samples, as previously reported [5].
Calculation of CD4 T-cell Decay
Through the CIHR/CTN 247 study, CD4 T-cell counts were prospectively combined with historical data from the medical charts for each study participant. A CD4 T-cell count versus date slope was created and a linear regression analysis performed to calculate annual change in CD4 count.
Plasma Assessment of IgG Titer and Plasma Levels of Lipopolysaccharide (LPS)
Plasma samples were obtained at the last study visit. Anti-CMV and anti-Epstein-Barr virus (EBV) IgG levels were measured using enzyme immunoassay test kits (GenWay Biotech, San Diego, CA, USA). Participants with plasma anti-CMV IgG levels greater than 0.1 IU/mL were considered CMV seropositive. Plasma LPS levels were measured by ELISA (Cusabio, Wuhan, China). Plasma IgM, IgG, and IgG1–4 were measured by ELISA (R&D systems, Minneapolis, MN, USA).
Statistical Analyses
Statistical analyses were conducted using SPSS 24.0 (IBM SPSS, Chicago, IL, USA) and GraphPad Prism 6.0 (GraphPad, La Jolla, CA, USA). Comparisons were conducted using nonparametric Mann-Whitney U test. Spearman rank correlation test was conducted to assess the associations between quantitative variables. An α level of 5% was used for statistical significance (P-value). Multivariate linear regression analyses were performed using SPSS 24.0.
Ethical Considerations
Ethical approval was obtained from the McGill University Health Centre ethics board, as well as all research ethics boards of participating and recruiting institutions. All study participants provided written consent.
RESULTS
Participant Characteristics
ECs had a median age of 47 years (range 26–72) and were followed for a median of 7.9 years (1.7–25.6). Out of 23 participants, 5 were female, and 10 (43%) had protective MHC-I HLA alleles HLA-B*27, -B*57, and/or -B*58. Median CD4 count at study visit was 650 cells/µL (290–1220), CD8 cells/µL count was 689 (211–1566), and CD4/CD8 ratio was 1 (0.6–3.1). All participants had plasma viral load <50 copies/mL, except 1 participant with a blip at 106 copies/mL. Five participants were CMV seronegative (undetectable anti-CMV IgG) and the 18 CMV seropositive participants had a median CMV IgG titer of 21.05 IU/mL (10.3–38.2) (Supplementary Table 1).
Anti-CMV IgG Titer Was Associated With CD4 Decay and Degree of Microbial Translocation in ECs
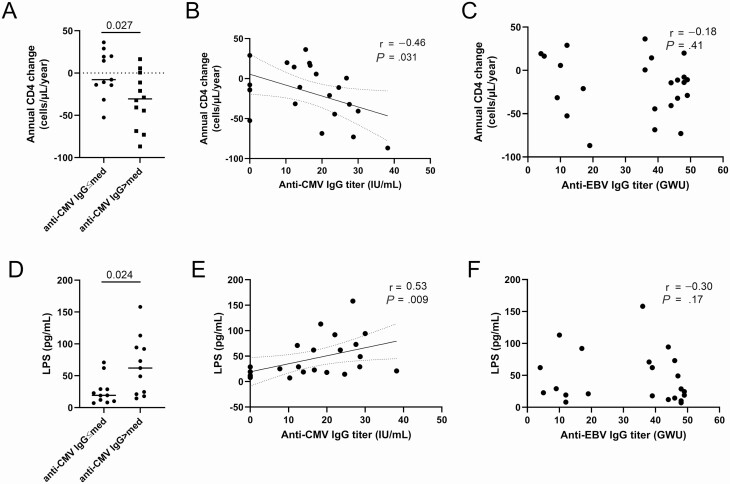
Over 7.9 years of follow-up, annual CD4 count change was not different between male and female (median −3.6 vs −21.1 cells/µL/year, P = .29), nor between ECs with or without protective HLA alleles (−14.3 vs −5.3 cells/µL/year, P = .48). Annual CD4 count change was negatively associated with CD4 count at the end of study follow-up (r = 0.55, P = .007) but not with CD8 count (r = 0.28, P = .19) nor CD4/CD8 ratio (r = 0.12, P = .60) (data not shown). No statistical difference in annual CD4 count change was observed between CMV seronegative and seropositive participants, although numbers of seronegative subjects were low (P = .801, Supplementary Figure 1). However, annual CD4 count change was lower in those with anti-CMV IgG titer greater than the median of 16.7 IU/mL (−30.6 vs −7.8 cells/µL/year, P = .027) (Figure 1A). Moreover, annual CD4 count change was negatively associated with anti-CMV IgG but not anti-EBV IgG levels at the end of study follow up (r = −0.46, P = .031; r = −0.18, P = .41, respectively) (Figure 1B, C, Supplementary Table 2). In CMV-seropositive ECs, anti-CMV IgG titers were associated with higher annual CD4 rate of decay (r = −0.72, P = .002, Supplementary Figure 2). Neither CD4 nor CD8 T-cell count were associated with anti-CMV IgG titers at study visit (r = −0.03, P = .91 and r = −0.39, P = .07, respectively). CD4 T-cell decay was not associated with plasma levels of total IgG, IgG2, 3, or 4 or immunoglobulin M (IgM) (Supplementary Table 2). However, CD4 count change was positively correlated with total IgG1 levels (r = 0.59, P = .004) and negatively with IgG2/IgG1 ratio (r = −0.46, P = .031). Moreover, anti-CMV IgG were negatively associated with total IgG1 (r = −0.57, P = .002) and positively with IgG2/IgG1 ratio (r = 0.52, P = .007) (Supplementary Table 2andSupplementary Figure 3). Multivariate analysis showed that association between annual CD4 decay and anti-CMV IgG titer was independent of age, sex, CD4/CD8 ratio, and expression of protective HLA alleles but not LPS level. Indeed, as we showed in non-EC PLWH [9], we observed that CMV seronegative ECs had lower levels of plasma LPS (19.3 vs 62 pg/mL, P = .02, Supplementary Table 1). Also, ECs with higher than the median anti-CMV IgG had higher levels of plasma LPS (62 vs 21 pg/mL, P = .024) (Figure 1D). Moreover, anti-CMV IgG, but not anti-EBV IgG nor IgM, IgG, and IgG1–4 titers were strongly associated with plasma LPS levels (r = 0.53, P = .009; r = −0.30, P = .17 respectively) (Figure 1E, F, Supplementary Table 3). However, plasma LPS levels were not associated with annual CD4 count change (r = −0.15, P = .51). Multivariate analysis demonstrated that the correlation between anti-CMV IgG titer and plasma levels of LPS were independent of age, sex, CD4/CD8 ratio, and protective HLA alleles.
Figure 1.
Anti-CMV IgG titer is associated with increased CD4 T-cell decay and bacterial translocation in EC. A, Annual CD4 T-cell count variation levels in EC with plasma anti-CMV-IgG levels lower or greater than the median (16.7 IU/mL) (Mann-Whitney U test). B, Annual CD4 T-cell variation is associated with elevated anti-CMV IgG titers (Spearman rank-order test). C, Plasma levels of LPS in ECs with plasma anti-CMV IgG levels lower or greater than the median (16.4 IU/mL) (Mann-Whitney U test). D, Plasma levels of LPS are associated with elevated anti-CMV IgG titers (Spearman’s rank-order test). Panels B and D display 95% confident interval with dotted lines. Abbreviations: CMV, cytomegalovirus; EBV, Epstein-Barr virus; EC, elite controllers; IgG, immunoglobulin G; LPS, lipopolysaccharide; med, median.
DISCUSSION
We observed an association between anti-CMV and CD4 T-cell decay in ECs, independent of age, sex, CD4/CD8 ratio, and presence of protective HLA alleles. However, this relationship was not independent of the microbial translocation maker LPS. In the general population, CMV coinfection has been associated with accelerated aging and increased risk of developing comorbidities. In PLWH not receiving ART, CMV coinfection and anti-CMV IgG levels were associated with increased inflammation and disease progression [11]. In ART-treated PLWH, CMV coinfection and anti-CMV IgG levels remained associated with higher risk of non-AIDS comorbidities [12]. Here we observed that annual CD4 count change was positively associated with total IgG1 and IgG2/IgG1. Moreover, anti-CMV IgG levels were also associated with IgG1 and IgG2/IgG1, indicating that CMV might favor B-cell activation and CD4 T-cell decay through different mechanisms. The relationship between CMV coinfection and disease progression is still unclear and conflicting results have been observed. However, our results support the hypothesis that the anti-CMV immune response is associated with CD4 T-cell decay in ECs.
CMV is able to productively infect intestinal epithelial cells and disrupt their tight junctions independently of HIV [10]. We previously showed that CMV coinfection and levels of anti-CMV IgG were associated with microbial translocation in ART-naive and ART-treated PLWH as well as HIV-uninfected controls [9]. Interestingly, our results show an association between anti-CMV IgG titer and plasma levels of LPS and CD4 decay in EC. Preferential infection and depletion of gut epithelial cells and mucosal CD4 T cells by CMV may contribute to the severity of epithelial gut damage and subsequent microbial translocation [4].
Our findings did not determine whether increased anti-CMV IgG levels are a cause or consequence of CD4 decay. In fact, decreasing CD4 T-cell count due to inflammation might favor CMV reactivation and increased production of anti-CMV IgG. On the other hand, CMV has been shown to induce gut barrier damage and inflammation in PLWH and the general aging population [9]. Hence, CMV could induce additional inflammation and in turn CD4 T-cell activation, leading to progressive T-cell loss.
Altogether, our results showed a negative association between anti-CMV IgG levels and annual CD4 T-cell count decay in ECs. CMV coinfection might contribute to CD4 T-cell decay and thus increased risk of developing non-AIDS comorbidities. Elevated CMV IgG levels may help determine the appropriate timing of ART initiation in ECs. Our findings stress the need for further studies assessing the implication of CMV in HIV disease progression.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. S. I. and R. R. performed the experiments, analyzed the data, wrote the first draft, and revised the final draft of the manuscript. S. I., R. R., J. L., S. K., B. F., J. O., X. P., and M. E. F. contributed to the experiments, data analysis, and critical review of the first and final drafts of the manuscript. J.-P. R. designed the study, contributed to data analysis, and critically reviewed the first and final drafts of the manuscript. N. F. B. contributed to the study design and critical review of the manuscript. All authors have read and approved the content of this manuscript.
Acknowledgments. The authors thank the study participants for their contributions as well as Angie Massicotte, Stéphanie Matte, Tsoarello Mabango, Louise Gilbert, Josée Girouard, and Dr Olfa Debbeche for study coordination and assistance. The authors also thank the Canadian cohort of HIV+ slow progressors physicians From Montreal, for recruitment and follow-up of study participants: R. Thomas, C. Milne, S. Lavoie, J. Friedman, F. Asselin, M. Boissonnault, P. J. Maziade, S. Lavoie, and M. E. Thériault (Clinique médicale l’Actuel); B. Lessard, M. A. Charron, E. Huchet, S. Poulin, D. Longpré, R. Pilarski, L. Charest, J.-G. Baril, A. Hamel, P. Coté, S. Dufrenes, and B. Trottier (Clinique médicale urbaine du Quartier Latin); L. Labrecque, C. Fortin, B. Deligne, V. Martel-Laferrière, and M. E. Goyer (Centre Hospitalier de l’Université de Montréal); R. Leblanc (Clinique medical Opus); J. Cox, L. P. Haraoui, M. Klein, B. Lebouché, A. de Pokomandy, and J. Szabo (McGill University Health Centre Chronic Viral Illness Service).
Financial support. This work was supported by the Fonds de la Recherche Québec-Santé (FRQ-S): Réseau SIDA/Maladies infectieuses and Thérapie cellulaire; the Canadian Institutes of Health Research (CIHR; grants HOP 103230 and PTJ 166049); the Vaccines & Immunotherapies Core of the CIHR Canadian HIV Trials Network (CTN; grant CTN 247); the Canadian Foundation for AIDS Research (CANFAR; grant 02-512); CIHR-funded Canadian HIV Cure Enterprise (CanCURE) Team Grant HB2-164064.
Potential conflicts of interest. C. T. declared grants from Merck and Gilead, and honorarium from Merck, Gilead, and ViiV, obtained outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ghosn J, Taiwo B, Seedat S, Autran B, Katlama C. HIV. Lancet 2018; 392:685–97. [DOI] [PubMed] [Google Scholar]
- 2. Okulicz JF, Marconi VC, Landrum ML, et al. ; Infectious Disease Clinical Research Program (IDCRP) HIV Working Group . Clinical outcomes of elite controllers, viremic controllers, and long-term nonprogressors in the US Department of Defense HIV natural history study. J Infect Dis 2009; 200:1714–23. [DOI] [PubMed] [Google Scholar]
- 3. Crowell TA, Gebo KA, Blankson JN, et al. ; HIV Research Network . Hospitalization rates and reasons among HIV elite controllers and persons with medically controlled HIV infection. J Infect Dis 2015; 211:1692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hunt PW, Brenchley J, Sinclair E, et al. Relationship between T-cell activation and CD4+ T-cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis 2008; 197:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. El-Far M, Kouassi P, Sylla M, et al. ; Investigators of the Canadian HIV+ Slow Progressor Cohort . Proinflammatory isoforms of IL-32 as novel and robust biomarkers for control failure in HIV-infected slow progressors. Sci Rep 2016; 6:22902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Christensen-Quick A, Vanpouille C, Lisco A, Gianella S. Cytomegalovirus and HIV persistence: pouring gas on the fire. AIDS Res Hum Retroviruses 2017; 33:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garg A, Gianella S, Nakazawa M, Trout R, Spector SA. Association of cytomegalovirus DNA and immunologic markers of cardiovascular disease. Open Forum Infect Dis 2019; 6:ofz113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gianella S, Morris SR, Tatro E, et al. Virologic correlates of anti-CMV IgG levels in HIV-1-infected men. J Infect Dis 2014; 209:452–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ramendra R, Isnard S, Lin J, et al. CMV seropositivity is associated with increased microbial translocation in people living with HIV and uninfected controls. Clin Infect Dis 2020; 71:1438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maidji E, Somsouk M, Rivera JM, Hunt PW, Stoddart CA. Replication of CMV in the gut of HIV-infected individuals and epithelial barrier dysfunction. PLoS Pathog 2017; 13:e1006202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patel EU, Gianella S, Newell K, et al. Elevated cytomegalovirus IgG antibody levels are associated with HIV-1 disease progression and immune activation. AIDS 2017; 31:807–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Letendre S, Bharti A, Perez-Valero I, et al. ; CNS HIV AntiRetroviral Therapy Effects Research (CHARTER) Group . Higher anti-cytomegalovirus immunoglobulin G concentrations are associated with worse neurocognitive performance during suppressive antiretroviral therapy. Clin Infect Dis 2018; 67:770–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.