Abstract
Background
Prior epidemiological and intervention studies have not been able to separate independent effects of dose, timing, and duration of aspirin use in colorectal cancer (CRC) chemoprevention. We examined aspirin-based CRC chemoprevention according to timing in the Nurses’ Health Study and Health Professionals Follow-Up Study.
Methods
The exposures include cumulative average dose and total duration of aspirin use in more than 10 years before follow-up started (remote period) and in the immediate 10 years before follow-up started (recent period). Cox models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for exposures and CRC risk.
Results
Aspirin use of longer than 10 years before follow-up started (HR = 0.88, 95% CI = 0.83 to 0.94) per 5-year increment and the immediate 10 years before follow-up started (HR = 0.90, 95% CI = 0.84 to 0.96) were similarly important in CRC chemoprevention, though a 5-year lag was required for a clear benefit in the recent period. In the remote period, the association was not dose dependent; compared with less than 0.5 standard-dose (325 mg) tablets per week; hazard ratios were 0.78 (95% CI = 0.63 to 0.98), 0.81 (95% CI = 0.72 to 0.91), and 0.74 (95% CI = 0.64 to 0.86) for doses of 0.5 to less than 1.5, 1.5 to less than 5, and 5 and more tablets per week, respectively. However, there was dose dependency in the recent period (with respective HR = 0.91, 95% CI = 0.79 to 1.06; HR = 0.87, 95% CI = 0.77 to 0.98; and HR = 0.76, 95% CI = 0.64 to 0.91).
Conclusions
A suggestive benefit necessitates at least 6-10 years and most clearly after approximately 10 years since initiation of aspirin. Remote use and use within the previous 10 years both contribute independently to decrease risk, though a lower dose may be required for a benefit with longer term use.
The global disease burden of colorectal cancer (CRC) is substantial (1,2). CRC accounted for 10% of all new cancer diagnoses and 9% of cancer deaths worldwide (1) and is estimated to be the third-most common incident cancer and the second leading cause of cancer death in the United States in 2020 (2). Aspirin (acetylsalicylic acid) is the most promising chemopreventive agent for CRC, with convincing evidence having emerged over the past 3 decades (3-8) since the hypothesis and initial observational studies and trials in this field were reported (3,9-15). In 2016, after systematic evidence reviews (16) and the balance of benefits and harms (17), the US Preventive Services Task Force (USPSTF) recommended low-dose aspirin for CRC primary prevention among US adults with specific age and cardiovascular risk profiles as a crucial first step (18).
Although the USPSTF recommendation reflects mounting evidence for the potential of aspirin in the complex landscape of CRC primary prevention, several important issues remained unsatisfactorily resolved entering the 2020s. Timing is a critical issue in aspirin-based CRC chemoprevention strategies (4,6,18). The concept of “delayed chemoprevention,” initially suggested by the Nurses’ Health Study (NHS) and Health Professionals Follow-up Study (HPFS) analyses (10,11), borne out over time, indicates that the observable benefit of aspirin on CRC would require approximately 10 or more years after initiation of aspirin use (4, 11, 12, 18–20). Aspirin may act at early stages of CRC carcinogenesis. However, important questions regarding timing remain, including if there is a time lag to demonstrate an apparent benefit, is continuing use necessary to reduce risk, or does it add benefit over remote use? If a benefit occurs in the remote period (eg, use before 10 years in the past), what is the dose and duration effect during this period? Is benefit of long-term aspirin use dose dependent? Is there heterogeneity in minimal effective dose across timing? Prior studies have not been able to adequately distinguish the effect of aspirin use according to timings (ie, in the remote and recent periods separately).
The NHS (21) and HPFS (22) longitudinal cohorts afford rich sources to consistently add high-quality evidence to this field (4,10,11,20,23). We thus comprehensively investigated the pivotal role of timing in aspirin-based primary CRC chemoprevention.
Methods
Study Population
This study was conducted using data from 2 ongoing US large cohort studies: the NHS and HPFS. The details of these cohorts have been described previously (21,24). The NHS was initiated in 1976 (21), when 121 700 female registered nurses aged 30 to 55 years were enrolled. The HPFS began in 1986 (24), enrolling 51 529 male health professionals aged between 40 and 75 years. Participants who were alive and free of cancer at the time when information on their aspirin use was first assessed were eligible for inclusion. Participants who reported any cancer or who reported colorectal adenoma removal were excluded from subsequent follow-up (Supplementary Methods, available online).
Assessment of Aspirin Use and Covariates
Duration and dose of aspirin use were first assessed in the NHS in 1980, with biennial updates thereafter except for 1986. In the HPFS, participants were first queried about duration and dose of aspirin use in 1986 and 1992, respectively, and with regular updates every 2 years. Considering it generally requires at least 10 years for newly onset adenomas to develop into CRC, we used total duration and cumulative average dose of aspirin use in the “remote periods” (ie, >10 years before follow-up periods) and “recent periods” (subsequent 10-year periods, ie, the immediate 10 years before follow-up periods), respectively, to assess long-term effects of aspirin in CRC chemoprevention (Figures 1 and 2; Supplementary Methods, available online). Covariates were selected a priori as potential confounders (Supplementary Methods, available online).
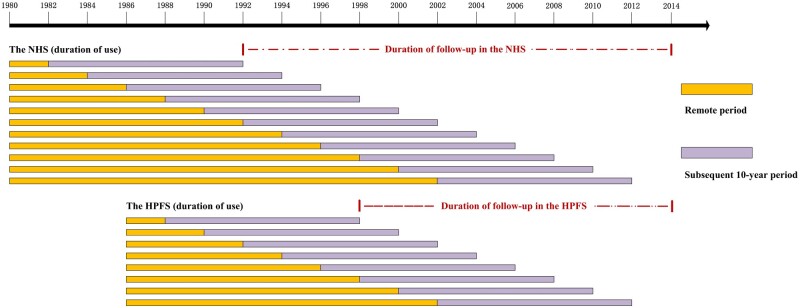
Figure 1.
Delayed effect of aspirin use and colorectal cancer (CRC) risk: timeline of analyses according to duration of use in the Nurses’ Health Study (NHS) and Health Professionals Follow-up Study (HPFS). While examining the delayed effects of duration of aspirin use in CRC chemoprevention: In the NHS, the first “remote period” was 1980-1982, and the first “subsequent 10-year period” was 1982-1992. The second “remote period” was 1980-1984, and the second “subsequent 10-year period” was 1984-1994. The last remote period was 1980-2002, then the last subsequent 10-year period was 2002-2012. Similarly, in the HPFS, the first remote period was 1986-1988, and the first subsequent 10-year period was 1988-1998. The last remote period was 1986-2002, and the last subsequent 10-year period was 2002-2012. The follow-up period was from the end of the first subsequent 10-year period (ie, 1992 in the NHS; 1998 in HPFS) to 2014.
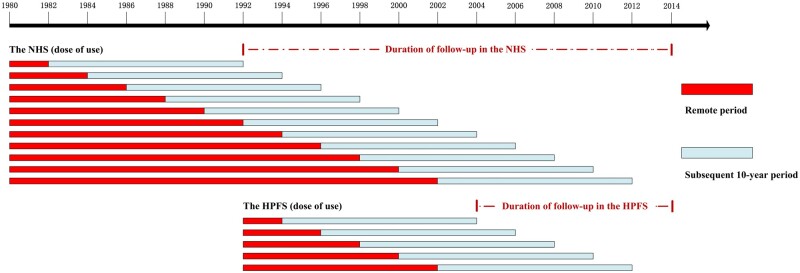
Figure 2.
Delayed effect of aspirin use and colorectal cancer (CRC) risk: timeline of analyses according to dose of use in the Nurses’ Health Study (NHS) and Health Professionals Follow-up Study (HPFS). While examining the delayed effects of dose of aspirin use in CRC chemoprevention: In the NHS, the first “remote period” was 1980-1982, and the first “subsequent 10-year period” was 1982-1992. The second remote period was 1980-1984, and the second subsequent 10-year period was 1984-1994. The last remote period was 1980-2002, then the last subsequent 10-year period was 2002-2012. Similarly, in the HPFS, the first remote period was 1992-1994, and the first subsequent 10-year period was 1994-2004. The last remote period was 1992-2002, and the last subsequent 10-year period was 2002-2012. The follow-up period was from the end of the first subsequent 10-year period (ie, 1992 in the NHS; 2004 in HPFS) to 2014.
Ascertainment of CRC Cases and Participant Deaths
CRC cases were ascertained via questionnaires, medical records, pathology reports, and state cancer registries. Participant deaths were confirmed through the National Death Index and next of kin or postal authorities (Supplementary Methods, available online).
Statistical Analysis
Person-years of follow-up accrued from the return date of the baseline questionnaire until the date of any cancer diagnosis reported, death recorded, loss to follow-up, diagnosis of colorectal adenoma, or follow-up completion (defined as 2014), whichever was earliest. The analysis was censored for those who had colorectal adenoma removed because this will alter the timing of the natural history for CRC.
Statistically significant heterogeneity by sex for each measure was not detected (Pheterogeneity > .33). We first performed separate analyses for each cohort. Pooled analyses were then combined by meta-analysis using the fixed-effect model. We used Cox proportional hazard models to estimate age- and multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) of the associations between duration and cumulative average dose of aspirin use in remote and recent periods and CRC risk (Supplementary Methods, available online). Given the entire follow-up time has been segmented in our primary analyses to investigate the pivotal role of timing, we are essentially not assuming proportional hazards for aspirin across all times but rather looking at individual time periods separately. The proportionality assumption for individual time periods was verified using interactions between the exposure of interest and the (log-) time scale.
In primary analyses, we presented hazard ratios and confidence intervals by categories of both duration and cumulative average dose of aspirin use and then by per 5-year increment in duration and per 2.5-standard tablets–per-week increase in dose, respectively. Tests for linear trend were performed by assigning the median values to categories of duration (in years) and dose (in 325-mg tablets per week) of aspirin use and modeling these values as continuous variables. Moreover, we mutually controlled for the above-mentioned time periods in separate multivariable-adjusted models due to the fact that, because use is correlated over time, recent and remote use could confound each other.
In secondary analyses, we further presented hazard ratios and confidence intervals for joint associations of remote and recent period aspirin use with CRC risk by using the collapsed categories. In duration analyses, remote and recent period aspirin use were dichotomized into low category (≤5 years) vs high category (>5 years), resulting in 4 categories (low remote to low recent, low remote to high recent, high remote to low recent, and high remote to high recent aspirin use). In dose analyses, the thresholds for dichotomization were defined as low (<1.5 tablets per week) vs high (≥1.5 tablets per week). We also assessed joint associations of duration and dose of aspirin use with risk of CRC in the remote and recent periods, first respectively and then combined. P values for interaction were calculated using the multiplication of mid-point of each low or high category in years (duration analyses) or in tablets (dose analyses).
For repeatedly measured variables, missing data were carried forward from the latest valid data in the previous follow-up cycle (allowing only 1 cycle). Missing indicators were created for the remaining categorical variables with no values and were included in the models when necessary.
We performed analyses using SAS statistical software (version 9.4; SAS Institute Inc), with 2-sided P values less than .05 indicating statistical significance.
Results
In this prospective study of 2 large cohorts, 123 816 eligible participants were included in duration analyses, with 2147 incident CRC cases documented. We included 113 582 eligible participants in dose analyses, with 1764 CRC cases documented. Variations across the exposures of interest were observed for age; race; body mass index; physical activity engagement; smoking status and pack-years of smoing; use of nonaspirin nonsteroidal antiinflammatory drugs and multivitamins; diagnoses of Type 2 diabetes; family history of CRC; Alternate Healthy Eating Index score; and intake of folate, vitamin D, calcium, alcohol, red or processed meat, total fiber, and total energy, etc (Tables 1 and 2; Supplementary Tables 1-4, available online).
Table 1.
Age-standardized participant characteristics according to remote period status of aspirin use in the pooled NHS and HPFSa
| Characteristics | Remote period duration of aspirin useb, y |
Remote period dose of aspirin usec, No. of 325-mg aspirin tablets/wk |
||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1-5 | 6-10 | >10 | <0.5 | 0.5 to <1.5 | 1.5 to <5 | ≥5 | |
| Remote period duration of aspirin useb, mean (SD), y | 0.0 (0.0) | 2.9 (1.0) | 7.6 (1.6) | 14.0 (2.4) | 0.1 (0.6) | 4.9 (3.0) | 5.8 (4.2) | 6.0 (4.5) |
| Recent period duration of aspirin used, mean (SD), y | 2.0 (2.9) | 4.4 (3.7) | 5.5 (3.7) | 6.7 (3.3) | 2.0 (2.9) | 3.5 (3.5) | 4.6 (3.7) | 6.0 (3.6) |
| Duration of nonaspirin NSAIDs use, mean (SD), y | 2.3 (3.0) | 3.1 (3.7) | 4.0 (4.4) | 6.0 (5.1) | 2.6 (3.1) | 3.5 (4.2) | 4.0 (4.4) | 3.8 (4.1) |
| Remote period dose of aspirin used (tablets/wk), mean (SD) | 0.0 (0.1) | 4.7 (5.5) | 4.9 (4.6) | 5.0 (3.3) | 0.0 (0.0) | 1.0 (0.3) | 3.0 (0.9) | 10.0 (6.5) |
| Recent period dose of aspirin usee (tablets/wk), mean (SD) | 1.1 (2.1) | 2.3 (3.1) | 2.6 (2.8) | 2.9 (2.4) | 1.1 (2.1) | 1.1 (1.7) | 1.9 (2.0) | 4.0 (3.9) |
| Dose of nonaspirin NSAIDs use (tablets/wk), mean (SD) | 1.8 (3.4) | 2.3 (3.8) | 2.7 (4.5) | 2.9 (4.8) | 1.5 (3.5) | 2.2 (3.8) | 2.1 (4.0) | 2.6 (4.8) |
| Age, mean (SD), y | 65.6 (9.0) | 66.9 (9.2) | 70.8 (8.5) | 74.9 (7.6) | 65.9 (9.0) | 69.6 (9.5) | 69.5 (9.1) | 68.8 (9.1) |
| White, % | 95.7 | 97.5 | 98.1 | 98.5 | 95.9 | 97.1 | 98.1 | 98.3 |
| BMI,f mean (SD), kg/m² | 26.1 (4.8) | 26.5 (5.0) | 26.8 (5.2) | 26.6 (5.1) | 26.1 (5.0) | 26.3 (4.9) | 26.5 (5.1) | 26.9 (5.5) |
| Physical activity,g mean (SD), METs-h/wk | 21.3 (26.1) | 21.1 (25.9) | 20.8 (25.6) | 20.4 (25.1) | 20.5 (25.5) | 22.2 (27.3) | 20.2 (25.1) | 18.5 (24.4) |
| Past smoking, % | 44.0 | 46.2 | 46.9 | 46.8 | 44.5 | 44.3 | 46.4 | 47.4 |
| Current smoking, % | 8.4 | 8.4 | 7.4 | 6.3 | 8.8 | 8.2 | 8.0 | 8.7 |
| Pack-years of smoking, mean (SD) | 12.3 (19.4) | 13.0 (19.5) | 12.6 (19.2) | 12.6 (18.5) | 12.5 (19.7) | 12.0 (18.7) | 12.5 (19.1) | 13.9 (20.4) |
| Type 2 diabetes, % | 5.9 | 6.9 | 8.1 | 10.2 | 6.3 | 6.6 | 7.7 | 9.0 |
| Family history of CRC, % | 15.5 | 16.0 | 15.6 | 16.1 | 15.7 | 15.4 | 16.5 | 16.6 |
| Multivitamin use, % | 48.2 | 55.3 | 59.8 | 64.9 | 48.3 | 52.1 | 58.8 | 58.9 |
| Total folate intake, mean (SD), μg/d | 624 (309) | 652 (322) | 706 (336) | 767 (320) | 614 (308) | 666 (329) | 674 (327) | 661 (327) |
| Total vitamin D, mean (SD), IU/d | 486 (363) | 520 (388) | 567 (424) | 695 (492) | 491 (375) | 563 (450) | 572 (446) | 549 (413) |
| Total calcium intake, mean (SD), mg/d | 1198 (570) | 1234 (585) | 1312 (607) | 1454 (603) | 1223 (581) | 1261 (591) | 1318 (611) | 1312 (622) |
| AHEI, mean (SD) | 51.5 (10.5) | 51.2 (10.6) | 51.8 (11.0) | 53.4 (10.9) | 51.7 (10.6) | 53.1 (10.7) | 51.9 (11.1) | 50.7 (10.7) |
| Alcohol, mean (SD), g/d | 6.0 (10.9) | 6.8 (11.6) | 7.2 (12.0) | 7.3 (11.6) | 5.5 (10.3) | 6.6 (11.3) | 6.5 (11.1) | 5.9 (11.0) |
| Red or processed meat, mean (SD), servings/wk | 4.6 (3.5) | 4.9 (3.6) | 5.0 (3.7) | 5.1 (3.5) | 4.5 (3.3) | 4.8 (3.5) | 4.8 (3.5) | 4.8 (3.6) |
| Total fiber, mean (SD), g/d, | 20.6 (6.3) | 20.6 (6.3) | 20.6 (6.3) | 20.5 (5.9) | 20.2 (6.1) | 20.8 (6.3) | 20.2 (6.0) | 19.9 (6.0) |
| Total calorie intake, mean (SD), kcal/d | 1742 (547) | 1781 (567) | 1794 (579) | 1776 (561) | 1714 (528) | 1768 (566) | 1758 (561) | 1746 (555) |
Updated information throughout follow-up was used to calculate the mean (SD) for continuous variables and percentage for categorical variables. All variables are age-standardized except age. AHEI = Alternate Healthy Eating Index score; BMI = body mass index; CRC = colorectal cancer; HPFS = Health Professionals Follow-up Study; METs = metabolic equivalent tasks; NHS = Nurses’ Health Study; NSAIDs = nonsteroidal antiinflammatory drugs.
Remote period duration of aspirin use: duration of regular aspirin use during every specific remote period (>10 years before every follow-up period, ie, began from 1980 [NHS] and 1986 [HPFS] and then was extended by every 2-year subsequent interval at a time until 2002).
Remote period dose of aspirin use: cumulative average aspirin use during every specific remote period (>10 years before every follow-up period, ie, began from 1980 [NHS] and 1992 [HPFS] and then was extended by every 2-year subsequent interval at a time until 2002).
Recent period duration of aspirin use: duration of regular aspirin use during every specific subsequent 10-year period after the remote periods.
Recent period dose of aspirin use: cumulative average aspirin use during every specific subsequent 10-year period after the remote periods.
BMI was calculated as weight in kilograms divided by the square of height in meters.
Hours of metabolic equivalent tasks.
Table 2.
Age-standardized participant characteristics according to recent period status of aspirin use in the pooled NHS and HPFSa
| Characteristics | Recent period duration of aspirin useb, y |
Recent period dose of aspirin usec, No. of 325-mg aspirin tablets/wk |
|||||
|---|---|---|---|---|---|---|---|
| 0 | 1-5 | 6-10 | <0.5 | 0.5 to <1.5 | 1.5 to <5 | ≥5 | |
| Remote period duration of aspirin used, mean (SD), y | 1.6 (3.0) | 3.4 (4.1) | 5.2 (4.8) | 1.9 (3.3) | 4.0 (4.3) | 4.5 (4.6) | 4.9 (4.9) |
| Recent period duration of aspirin usee, mean (SD), y | 0.0 (0.0) | 2.9 (1.0) | 8.2 (1.7) | 0.4 (1.5) | 5.7 (2.8) | 6.3 (2.9) | 6.0 (3.2) |
| Duration of nonaspirin NSAIDs use, mean (SD), y | 2.9 (3.5) | 3.3 (3.9) | 3.5 (4.1) | 3.1 (3.6) | 3.9 (4.5) | 3.5 (4.0) | 3.2 (3.5) |
| Remote period dose of aspirin usee (tablets/wk), mean (SD) | 1.3 (3.1) | 2.7 (4.2) | 4.5 (5.4) | 1.4 (3.1) | 2.2 (2.8) | 3.4 (3.7) | 7.0 (8.1) |
| Recent period dose of aspirin usec (tablets/wk), mean (SD) | 0.0 (0.0) | 3.0 (3.1) | 3.2 (2.7) | 0.0 (0.1) | 1.0 (0.3) | 2.8 (0.9) | 7.5 (3.5) |
| Dose of nonaspirin NSAIDs use (tablets/wk), mean (SD) | 1.8 (3.7) | 2.3 (3.9) | 2.6 (4.0) | 1.6 (3.7) | 2.3 (4.0) | 2.0 (4.0) | 2.3 (4.5) |
| Age, mean (SD), y | 65.7 (9.2) | 67.6 (9.2) | 69.9 (9.0) | 66.2 (9.3) | 71.2 (8.6) | 68.7 (9.1) | 67.1 (8.9) |
| White, % | 96.0 | 97.2 | 97.9 | 96.2 | 97.4 | 98.0 | 98.3 |
| BMI,f mean (SD), kg/m² | 26.2 (5.0) | 26.5 (5.0) | 26.5 (4.9) | 26.2 (5.1) | 26.3 (4.9) | 26.5 (5.1) | 27.0 (5.5) |
| Physical activity,g mean (SD), METs-h/wk | 19.8 (25.0) | 21.0 (26.0) | 22.6 (26.8) | 19.3 (24.7) | 22.6 (27.2) | 20.4 (25.3) | 19.0 (24.3) |
| Past smoking, % | 44.0 | 45.9 | 46.5 | 44.6 | 45.9 | 46.6 | 46.9 |
| Current smoking, % | 8.7 | 7.8 | 7.5 | 8.7 | 6.8 | 8.2 | 9.8 |
| Pack-years of smoking, mean (SD) | 12.2 (19.3) | 12.5 (19.2) | 12.8 (19.4) | 12.3 (19.4) | 11.4 (18.1) | 13.0 (19.6) | 15.0 (21.3) |
| Type 2 diabetes, % | 5.7 | 7.1 | 7.9 | 6.0 | 7.2 | 8.2 | 9.0 |
| Family history of CRC, % | 15.5 | 15.6 | 16.0 | 15.7 | 15.3 | 16.7 | 16.9 |
| Multivitamin use, % | 44.3 | 53.1 | 63.4 | 45.1 | 58.8 | 61.5 | 59.3 |
| Total folate intake, mean (SD), μg/d | 605 (300) | 652 (318) | 715 (339) | 605 (303) | 708 (330) | 675 (329) | 640 (321) |
| Total vitamin D, mean (SD), IU/d | 484 (379) | 519 (389) | 579 (430) | 493 (392) | 596 (458) | 561 (421) | 524 (393) |
| Total calcium intake, mean (SD), mg/d | 1200 (571) | 1238 (583) | 1299 (608) | 1221 (579) | 1329 (603) | 1317 (615) | 1276 (614) |
| AHEI, mean (SD) | 51.1 (10.4) | 51.4 (10.6) | 52.2 (11.1) | 51.4 (10.5) | 53.4 (11.1) | 51.7 (10.9) | 50.3 (10.6) |
| Alcohol, mean (SD), g/d | 5.5 (10.4) | 6.5 (11.2) | 7.8 (12.5) | 5.3 (10.0) | 6.9 (11.4) | 6.5 (11.2) | 6.0 (11.1) |
| Red or processed meat, mean (SD), servings/wk | 4.7 (3.4) | 4.9 (3.6) | 5.0 (3.8) | 4.6 (3.3) | 4.8 (3.5) | 4.7 (3.5) | 4.8 (3.6) |
| Total fiber, mean (SD), g/d, | 20.1 (6.0) | 20.6 (6.2) | 21.1 (6.5) | 19.9 (5.9) | 20.9 (6.3) | 20.3 (6.1) | 19.8 (6.0) |
| Total calorie intake, mean (SD), kcal/d | 1732 (534) | 1770 (563) | 1802 (588) | 1717 (524) | 1771 (569) | 1748 (560) | 1746 (555) |
Updated information throughout follow-up was used to calculate the mean (SD) for continuous variables and percentage for categorical variables. All variables are age standardized except age. AHEI = Alternate Healthy Eating Index score; BMI = body mass index; CRC = colorectal cancer; HPFS = Health Professionals Follow-up Study; METs = metabolic equivalent tasks; NHS = Nurses’ Health Study; NSAIDs = nonsteroidal anti-inflammatory drugs.
Recent period duration of aspirin use: duration of regular aspirin use during every specific subsequent 10-year period after the remote periods.
Recent period dose of aspirin use: cumulative average aspirin use during every specific subsequent 10-year period after the remote periods.
Remote period duration of aspirin use: duration of regular aspirin use during every specific remote period (>10 years before every follow-up period, ie, began from 1980 [NHS] and 1986 [HPFS] and then was extended by every 2-year subsequent interval at a time until 2002).
Remote period dose of aspirin use: cumulative average aspirin use during every specific remote period (>10 years before every follow-up period, ie, began from 1980 [NHS] and 1992 [HPFS] and then was extended by every 2-year subsequent interval at a time until 2002).
BMI was calculated as weight in kilograms divided by the square of height in meters.
Hours of metabolic equivalent tasks.
When exploring the association between duration of aspirin use and CRC risk according to timing in fully adjusted pooled analyses, compared with participants who never used aspirin in the remote period, longer duration of aspirin use in the remote period was associated with a decreased CRC risk (HR = 0.88, 95% CI = 0.83 to 0.94 per 5-year increment). Similarly, in the recent period, compared with participants who reported no aspirin use in this period, longer duration of use was associated with lower CRC risk (HR = 0.90, 95% CI = 0.84 to 0.96, per 5-year increment). The apparent (statistically significant) benefit of aspirin was observed in the 1-5 years of use in the remote period and 6 or more years of use in the recent period (Table 3; Supplementary Tables 5 and 6, available online).
Table 3.
Remote period duration of aspirin use,a recent period duration of aspirin use,b and CRC risk in the pooled NHS and HPFS
| Pooled NHS and HPFS | Regular aspirin use, y |
P trend c | Per 5-year increment HR (95% CI) | P trend d | |||
|---|---|---|---|---|---|---|---|
| 0 | 1-5 | 6-10 | >10 | ||||
| Remote period duration of aspirin usea | |||||||
| Median values | 0 | 2 | 8 | 14 | — | — | — |
| No. of CRC cases (2147 in total) | 1050 | 612 | 353 | 132 | — | — | — |
| Age-adjusted model, HR (95% CI)e | 1 (Reference) | 0.79 (0.71 to 0.87) | 0.67 (0.59 to 0.75) | 0.70 (0.58 to 0.86) | <.001 | 0.83 (0.79 to 0.88) | <.001 |
| MV-adjusted model, HR (95% CI)f | 1 (Reference) | 0.79 (0.72 to 0.88) | 0.68 (0.60 to 0.77) | 0.72 (0.60 to 0.88) | <.001 | 0.84 (0.79 to 0.90) | <.001 |
| MVf + recent period duration of aspirin useb adjusted model, HR (95% CI) | 1 (Reference) | 0.83 (0.75 to 0.92) | 0.73 (0.63 to 0.83) | 0.79 (0.65 to 0.97) | <.001 | 0.88 (0.83 to 0.94) | <.001 |
| Recent period duration of aspirin useb | |||||||
| Median values | 0 | 2 | 8 | — | — | — | — |
| No. of CRC cases (2147 in total) | 855 | 571 | 721 | — | — | — | — |
| Age-adjusted model, HR (95% CI)e | 1 (Reference) | 0.88 (0.79 to 0.97) | 0.72 (0.65 to 0.80) | — | <.001 | 0.83 (0.78 to 0.88) | <.001 |
| MV-adjusted morel, HR (95% CI)f | 1 (Reference) | 0.88 (0.79 to 0.99) | 0.75 (0.67 to 0.83) | — | <.001 | 0.84 (0.79 to 0.90) | <.001 |
| MVf + remote period duration of aspirin usea adjusted model, HR (95% CI) | 1 (Reference) | 0.94 (0.84 to 1.05) | 0.84 (0.74 to 0.94) | — | .002 | 0.90 (0.84 to 0.96) | .002 |
Remote period duration of aspirin use: duration of regular aspirin use during every specific remote period (>10 years before every follow-up period, ie, began from 1980 [NHS] and 1986 [HPFS] and then was extended by every 2-year subsequent interval at a time until 2002). AHEI = Alternate Healthy Eating Index score; BMI = body mass index; CI = confidence interval; CRC = colorectal cancer; HPFS = Health Professionals Follow-up Study; HR = hazard ratio; MV = multivariable; NHS = Nurses’ Health Study; NSAIDs = nonsteroidal antiinflammatory drugs.
Recent period duration of aspirin use: duration of regular aspirin use during every specific subsequent 10-year period after the remote periods.
Adjusted for age and follow-up cycle in the follow-up period.
Adjusted for age, follow-up cycle, sex, race, pack-years of smoking, physical activity, BMI, alcohol consumption, AHEI, regular use of nonaspirin NSAIDs, family history of CRC, history of diabetes mellitus, screening colonoscopy or sigmoidoscopy in the past 2 years, multivitamin use, total calorie intake, red or processed meat intake, fiber intake, folate intake, calcium intake, and vitamin D intake in the follow-up period.
P trend was calculated using the mid-point of each category of aspirin use in years.
f P trend was the P value for variables modeled as continuous.
When examining the association between dose of aspirin use and CRC risk according to timing in fully adjusted pooled analyses, in both the remote and recent periods, larger dose in the same period was associated with a decreased risk of CRC. In the remote period, the association was not dose dependent; compared with participants who used aspirin with a cumulative average dose of less than 0.5 standard-dose tablets per week, hazard ratios were 0.78 (95% CI = 0.63 to 0.98), 0.81 (95% CI = 0.72 to 0.91), and 0.74 (95% CI = 0.64 to 0.86) for doses of 0.5 to less than 1.5, 1.5 to less than 5, and 5 and more tablets per week, respectively. However, there was dose dependency in the recent period (with respective HRs of 0.91, 95% CI = 0.79 to 1.06; 0.87, 95% CI = 0.77 to 0.98; and 0.76, 95% CI = 0.64 to 0.91). It is noteworthy that results were statistically significant for those who used 0.5 and more tablets per week in the remote period and were only robust for those used 1.5 and more tablets per week in the recent period (Table 4; Supplementary Tables 7-10, available online).
Table 4.
Remote period dose of aspirin use,a recent period dose of aspirin use,b and CRC risk in the pooled NHS and HPFS
| Pooled NHS and HPFS | No. of 325-mg aspirin tablets/wk (cumulative mean) |
P trend c | Per 2.5-standard tablets/wk increment HR (95% CI) | P trend d | |||
|---|---|---|---|---|---|---|---|
| <0.5 | 0.5 to <1.5 | 1.5 to <5 | ≥5 | ||||
| Remote period dose of aspirin usea | |||||||
| Median values | 0 | 1.00 | 2.90 | 7.54 | — | — | — |
| No. of CRC cases (1764 in total) | 875 | 88 | 531 | 270 | — | — | — |
| Age-adjusted model, HR (95% CI)e | 1 (Reference) | 0.77 (0.61 to 0.96) | 0.76 (0.68 to 0.85) | 0.67 (0.58 to 0.77) | <.001 | 0.92 (0.89 to 0.95) | <.001 |
| MV-adjusted model, HR (95% CI)f | 1 (Reference) | 0.77 (0.62 to 0.96) | 0.77 (0.69 to 0.87) | 0.68 (0.59 to 0.78) | <.001 | 0.93 (0.90 to 0.96) | <.001 |
| MVf + recent period aspirin useb adjusted model, HR (95% CI) | 1 (Reference) | 0.78 (0.63 to 0.98) | 0.81 (0.72 to 0.91) | 0.74 (0.64 to 0.86) | <.001 | 0.95 (0.92 to 0.98) | .001 |
| Recent period dose of aspirin useb | |||||||
| Median values | 0 | 1.05 | 2.63 | 6.25 | — | — | — |
| No. of CRC cases (1764 in total) | 820 | 247 | 524 | 173 | — | — | — |
| Age-adjusted model, HR (95% CI)e | 1 (Reference) | 0.83 (0.72 to 0.96) | 0.79 (0.71 to 0.88) | 0.69 (0.58 to 0.81) | <.001 | 0.88 (0.84 to 0.93) | <.001 |
| MV-adjusted model, HR (95% CI)f | 1 (Reference) | 0.86 (0.74 to 1.00) | 0.80 (0.71 to 0.90) | 0.68 (0.58 to 0.80) | <.001 | 0.88 (0.83 to 0.92) | <.001 |
| MVf + remote period aspirin usea adjusted model, HR (95% CI) | 1 (Reference) | 0.91 (0.79 to 1.06) | 0.87 (0.77 to 0.98) | 0.76 (0.64 to 0.91) | .001 | 0.91 (0.87 to 0.97) | .001 |
Remote period dose of aspirin use: cumulative average dose of aspirin use during every specific remote period (>10 years before every follow-up period, ie, began from 1980 [NHS] and 1992 [HPFS] and then was extended by every 2-year subsequent interval at a time until 2002). AHEI = Alternate Healthy Eating Index score; BMI = body mass index; CI = confidence interval; CRC = colorectal cancer; HPFS = Health Professionals Follow-up Study; HR = hazard ratio; MV = multivariable; NHS = Nurses’ Health Study; NSAIDs = nonsteroidal antiinflammatory drugs.
Recent period dose of aspirin use: cumulative average dose of aspirin use during every specific subsequent 10-year period after the remote periods.
Adjusted for age and follow-up cycle in the follow-up period.
Adjusted for age, follow-up cycle, sex, race, pack-years of smoking, physical activity, BMI, alcohol consumption, AHEI, regular use of nonaspirin NSAIDs, family history of CRC, history of diabetes mellitus, screening colonoscopy or sigmoidoscopy in the past 2 years, multivitamin use, total calorie intake, red or processed meat intake, fiber intake, folate intake, calcium intake, and vitamin D intake in the follow-up period.
P trend was calculated using the mid-point of each category of aspirin use in tablets per week.
P trend was the P value for variables modeled as continuous.
We then explored joint association of remote and recent period aspirin use with CRC risk according to duration and dose. In fully adjusted pooled analyses, the duration of aspirin use in remote and recent periods seemed to have independent associations in CRC chemoprevention. The benefit from the remote period was sustained even after a decade, and those who had longer duration of aspirin use in both periods had a lower risk than in each period singly (Tables 5 and 6; Supplementary Tables 11 and 12, available online).
Table 5.
Joint associations of remote perioda and recent periodb duration of aspirin use with CRC risk in the NHS and HPFS
| Pooled NHS and HPFS | Joint associations according to duration of aspirin use and timing |
P interaction d | |||
|---|---|---|---|---|---|
| Low to lowc | Low to highc | High to lowc | High to highc | ||
| No. of CRC cases (2147 in total) | 1225 | 437 | 201 | 284 | |
| Age-adjusted model, HR (95% CI)e | 1 (Reference) | 0.80 (0.72 to 0.90) | 0.78 (0.67 to 0.91) | 0.65 (0.57 to 0.74) | .72 |
| MV-adjusted model, HR (95% CI)f | 1 (Reference) | 0.82 (0.73 to 0.92) | 0.79 (0.68 to 0.92) | 0.67 (0.58 to 0.77) | .75 |
Remote period duration of aspirin use: duration of regular aspirin use during every specific remote period (>10 years before every follow-up period, ie, begin from 1980 [NHS] and 1986 [HPFS], and then was extended by every 2-year subsequent interval at a time, until 2002). AHEI = Alternate Healthy Eating Index score; BMI = body mass index; CI = confidence interval; CRC = colorectal cancer; HPFS = Health Professionals Follow-up Study; HR = hazard ratio; MV = multivariable; NHS = Nurses’ Health Study; NSAIDs = nonsteroidal antiinflammatory drugs.
Recent period duration of aspirin use: duration of regular aspirin use during every specific subsequent 10-year period after the remote periods.
Adjusted for age and follow-up cycle in the follow-up period.
Adjusted for age, follow-up cycle, sex, race, pack-years of smoking, physical activity, BMI, alcohol consumption, AHEI, regular use of nonaspirin NSAIDs, family history of CRC, history of diabetes mellitus, screening colonoscopy or sigmoidoscopy in the past 2 years, multivitamin use, total calorie intake, red or processed meat intake, fiber intake, folate intake, calcium intake, and vitamin D intake in the follow-up period.
Remote period duration of aspirin use and recent period duration of aspirin use were dichotomized into low category (≤5 years) vs high category (>5 years), resulting in 4 categories (low to low, low to high, high to low, and high to high, ie, low remote to low recent, low remote to high recent, high remote to low recent, and high remote to high recent duration of aspirin use).
P interaction was calculated using the multiplication of mid-point of each low or high category of duration of aspirin use in years.
Table 6.
Joint associations of remote perioda and recent periodb dose of aspirin use with CRC risk in the NHS and HPFS
| Pooled NHS and HPFS | Joint associations according to dose of aspirin use and timing |
P interaction d | |||
|---|---|---|---|---|---|
| Low to lowc | Low to highc | High to lowc | High to highc | ||
| No. of CRC cases (1746 in total) | 729 | 234 | 338 | 463 | |
| Age-adjusted model, HR (95% CI)e | 1 (Reference) | 0.84 (0.72 to 0.97) | 0.77 (0.68 to 0.88) | 0.68 (0.61 to 0.77) | .47 |
| MV-adjusted model, HR (95% CI)f | 1 (Reference) | 0.84 (0.72 to 0.97) | 0.79 (0.69 to 0.90) | 0.69 (0.61 to 0.77) | .56 |
Remote period dose of aspirin use: cumulative average dose of aspirin use during every specific remote period (>10 years before every follow-up period, ie, begin from 1980 [NHS] and 1992 [HPFS], and then was extended by every 2-year subsequent interval at a time, until 2002). AHEI = Alternate Healthy Eating Index score; BMI = body mass index; CI = confidence interval; CRC = Colorectal cancer; HPFS = Health Professionals Follow-up Study; HR = hazard ratio; MV = multivariable; NHS = Nurses’ Health Study; NSAIDs = nonsteroidal antiinflammatory drugs.
Recent period dose of aspirin use: cumulative average dose of aspirin use during every specific subsequent 10-year period after the remote periods.
Adjusted for age and follow-up cycle in the follow-up period.
Adjusted for age, follow-up cycle, sex, race, pack-years of smoking, physical activity, BMI, alcohol consumption, AHEI, regular use of nonaspirin NSAIDs, family history of CRC, history of diabetes mellitus, screening colonoscopy or sigmoidoscopy in the past 2 years, multivitamin use, total calorie intake, red or processed meat intake, fiber intake, folate intake, calcium intake, and vitamin D intake in the follow-up period.
Remote period dose of aspirin use and recent period dose of aspirin use were dichotomized into low category (<1.5 tablets per week) vs high category (≥1.5 tablets per week), resulting in 4 categories (low to low, low to high, high to low, and high to high, ie, low remote to low recent, low remote to high recent, high remote to low recent, and high remote to high recent dose of aspirin use).
P interaction was calculated using the multiplication of mid-point of each low/high category of dose of aspirin use in tablets per week.
We examined joint association of duration and dose of aspirin use with CRC risk according to timing. In fully adjusted pooled analyses, in the remote period, participants reported high duration and low dose of aspirin use and those who reported low duration and high dose had a similarly decreased risk of CRC, with the lowest CRC risk observed among those used both high duration and dose. Interestingly, in the recent period, participants who reported low duration and high dose might get more benefit from aspirin compared with those who used high duration and low dose at the same time (Supplementary Tables 13 and 14, available online).
We further explored joint association of overall status (combined duration and dose) and timing of aspirin use with CRC risk based on thresholds of duration and dose derived from results in Tables 3 and 4. In fully adjusted pooled analyses, the overall status of using aspirin in the remote period seemed to have independent effects, and the benefit from the remote period was sustained even after a decade. This combined overall status in the recent period tended to also have an independent effect (Supplementary Table 15, available online).
Discussion
Defining the optimum treatment duration and the lowest effective dose is of critical importance for aspirin-based CRC chemoprevention (4,6,18). In this large prospective cohort study, we rigorously examined the duration- and dose-dependent associations between aspirin use and CRC risk in the context of timing. To the best of our knowledge, we presented the first study that completely separated effect of aspirin exposure in the remote period (>10 years before follow-up started) from that in the recent period (immediate 10 years before follow-up started) and disentangled their mixed effect in the subsequent independent follow-up period.
In the 1990s, findings from the NHS and HPFS analyses (10,11) initially suggested the concept of “delayed chemoprevention” of at least 10 years in aspirin-based CRC primary prevention (4). This concept, indicating the importance of timing in this association, explained the null findings from early trials (12,25,26) and was borne out over time (4) by follow-up studies with updated data (19,20,23,27) and many other high-quality observational studies and trials (16,28,29). In 2016, the USPSTF, after a systemic review of prior evidence (16), recommended low-dose aspirin for CRC primary prevention (among adults with certain age and risk profiles) with an emphasis on keeping at least a decade of regular use (18). Of note, among the high-risk population (eg, those with Lynch syndrome), this latency may be shortened (30-33). In 2020, the UK National Institute for Health and Care Excellence also advised using daily aspirin for CRC prevention among people with Lynch syndrome in the latest guidance (34), though optimal dose remained to be determined.
In this study, we reported that the apparent benefit of aspirin was duration and dose dependent in both periods, appearing generally after 1-5 years of use in the remote period and after 6 or more years of use in the recent period. Interestingly, the dose requirement appeared to vary, with 0.5 and more standard-dose tablets per week (≥23 mg/d) sufficient in the remote period, but 1.5 and more standard-dose tablets per week (≥70 mg/d) in the recent period.
Collectively, we observed no association between aspirin use and CRC risk within up to 5 years and a suggestive benefit after 6-10 years of use. A clearer benefit was detected only after 10 years of usage, and it persisted regardless of continuing use or not. Our duration-dependent finding in the average-risk population adds to the existing evidence in support of an approximate 10-year latency before benefit of aspirin-based chemoprevention could be observed among men (35) and is generally consistent with the suggested similar latency among women in previous studies (≥10 years) (20,27), the systemic reviews of randomized trials and observational studies for both sexes combined (≥10 years) (16,19,29), and latest updates afterwards (≥5 years) (23,36).
Prior evidence generally supported that statistically significant CRC risk reduction might be achieved with long-term use of dose equivalents as low as 70-75 mg/d (16,18,20,23,36), 100 mg per alternate day (27), or higher (19). It is still controversial whether benefit of long-term aspirin use is dose dependent (16,37). Nonetheless, few trials directly compared different doses. Our results, indicating potential heterogeneity in minimal effective dose across timing (remote period: not dose dependent; recent period: dose dependent, with a minimum effective dose of 70 mg/d), was also not completely consistent with prior findings and recommendations from the USPSTF Statement (18). Interestingly, those with lower duration and high dose and with high duration and low dose received largely similar benefits. In the 10 years before CRC diagnosis, it is more likely that an adenoma may already be present, so the apparent dose requirement difference (low in remote period, high in the recent period) may reflect whether the precursor lesion is present and how advanced it may be.
Clinical decision making is typically guided by indications of use (eg, cardiovascular disease), risk of developing CRC, and risk to benefit profile (18). The USPSTF recommends initiating low-dose (81 mg/d) aspirin use for CRC primary prevention in adults aged 50-59 years or 60-69 years who have a 10% or greater 10-year CVD risk, are not at increased risk for bleeding, have a life expectancy of at least 10 years, and are willing to take low-dose aspirin daily for at least 10 years (18). The existing evidence is insufficient among those aged 50 years old and younger or 70 years and older (18). Of note, the most recent large trial of 100 mg/d aspirin use has suggested increased risks of mortality and late-stage cancers among adults predominantly aged 70 years and older (38-40). Our findings may have provided new initial clues of practical importance in altering patient management: for example, the potential benefit of initiating aspirin use at even lower doses (eg, 23-70 mg/d) among persons with a life expectancy exceeding 15-20 years, and the possibility of using lower dose (70 mg/d) among persons (especially for those ≥70 years of age) that have already used aspirin for at least 10 years.
Mechanisms driving the potential of aspirin in CRC chemoprevention remain inconclusive, with multiple interrelated pathways (eg, prostaglandin synthesis and catabolism, platelet-mediated effects, WNT–β-catenin signaling, inflammatory and immune responses) having been suggested (3,5,8). Most CRCs develop from colorectal adenomas, and newly onset adenomas generally require at least 10 years to develop into carcinoma (4). The effects of aspirin, starting from a low dose, may be predominantly explained by regulation of platelet-induced phenotypic switching of cells involved in colorectal neoplastic transformation (3). This reflects that low-dose aspirin may be operative at early stages of colorectal carcinogenesis (3,4), which has also been supported by most of the observational evidence and prevention trials linking low-dose aspirin use to decreased risk or recurrence of colorectal adenomas in average-risk (15,41) and high-risk (42-46) populations, albeit a few mixed findings exist (44,47). However, the mechanisms driving the time and dose dependence of the observed effects remain to be explored.
Our study represents one of the largest prospective studies to investigate this topic. By setting “hard” thresholds on timeline, we were able to rigorously examine the effects of aspirin use in remote and recent periods in the natural history of CRC and account for their independent and joint effects for the first time. Importantly, this also allowed us to reduce confounding of recent aspirin use from past aspirin use. Major strengths of this study also include detailed assessments of exposures (prospectively updated aspirin intake data throughout follow-up permits precise assessments of its benefits at a broad range of doses, durations, and timings), validated time-varying information on a wide range of covariates, minimized socioeconomic confounding, and satisfying data quality and internal validity, etc. Our study also has limitations. First, as an observational study, our results are not as definitive as those of randomized clinical trials designed for CRC primary prevention purpose. However, it is not likely to be feasible to consider such trials with adequate follow-up time in the context of “10-year delayed chemoprevention” suggested by prior analyses, the high prevalence of aspirin use required in various clinical practices, and the ethical concerns (given the efficacy of current colonoscopy screening practices). Second, demographic homogeneity of our study participants (all health-care professionals and predominantly White) may limit the generalizability of the current findings to diverse populations. Lastly, we were not able to individually examine nonaspirin nonsteroidal antiinflammatory drugs by using the current analytic strategy because their quantity of use was not assessed with adequate follow-up time.
Other challenges regarding use of aspirin in CRC chemoprevention should be mentioned. First, the hints that aspirin-mediated reductions in CRC risk may differ by subsites (proximal or distal colon) (37), molecular subtypes (48-50), genetic variants (51), inflammatory status (52,53), immune response in the tumor microenvironment (54), high- or average-risk groups (31-33), bodyweight or body mass index (55,56), smoking status (55), and race (57) add further complexity. Second, the potential dose or duration-response relationships observed in these associations need to be validated in randomized trials. Lastly, although low-dose aspirin generally has the most favorable risk to benefit profile (58), the absolute bleeding risk may vary considerably by patient (59). The 1-dose or duration-fits-all strategy in aspirin-based CRC chemoprevention may not be optimal, and therefore more individualized chemoprevention strategies are warranted in the context of precision medicine.
In conclusion, the present large prospective cohort study supports a delayed effect of aspirin use in CRC chemoprevention. A suggestive benefit necessitates at least 6-10 years and most clearly after approximately 10 years since initiation of aspirin. Remote use (not dose dependent) and use within the previous 10 years (dose dependent) both contribute independently to decreased CRC risk, though a lower dose may be required for a benefit with longer term use. Our findings may have provided new initial clues of practical importance in altering patient management, including the potential benefit of initiating using aspirin at even lower doses (eg, 23-70 mg/d) among persons with a life expectancy exceeding 15-20 years, and the possibility of using lower doses (70 mg/d) among persons (especially for those aged ≥70 years) who have already used aspirin for at least 10 years. The decision making should be guided in the context of timing.
Funding
The NHS and HPFS were supported by grants UM1 CA186107, UM1 CA176726, UM1 CA167552, P01 CA87969, U01 CA167552, and R03 CA223619 from the National Institutes of Health (NIH). ATC was supported by NIH grants R01 CA137178, R35 CA253185, and K24 DK098311 and by the Damon Runyon Cancer Research Foundation. ELG was supported by a grant from the World Cancer Research Fund.
Notes
Role of the funders: The funding sources played no role in the study design, data collection, data analysis, and interpretation of results, or the decisions made in preparation and submission of the article.
Disclosures: JAM declares institutional research funding from Boston Biomedical and consulting for Ignyta, Taiho Pharmaceutical, and Cota, outside the submitted work. ATC declares research funding from Bayer and consulting for Bayer and Pfizer, outside the submitted work. Other authors declare no conflict of interest.
Author contributions: YZ and ELG had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: ELG, YZ. Acquisition, analysis, or interpretation of data: YZ. Drafting of the manuscript: YZ. Critical revision of the manuscript for important intellectual content: ELG, ATC, JAM. Statistical analysis: YZ. Obtained funding: ELG, ATC. Administrative, technical, or material support: ELG, ATC, JAM. Supervision: ELG.
Acknowledgements: The authors thank all participants and staff of the Nurses' Health Study and Health Professionals Follow-up Study for their contributions to this research. We are grateful for help from the following state cancer registries: Alabama, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, Washington, and Wyoming. The authors assume full responsibility for analyses and interpretation of these data.
Data Availability
Data, the statistical code, questionnaires, and technical processes are available from the corresponding author at egiovann@hsph.harvard.edu.
Supplementary Material
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2020. CA A Cancer J Clin. 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 3. Patrignani P, Patrono C.. Aspirin and cancer. J Am Coll Cardiol. 2016;68(9):967–976. [DOI] [PubMed] [Google Scholar]
- 4. Giovannucci E. Aspirin and delayed chemoprevention of colorectal cancer. Clin Chem. 2018;64(11):1668–1669. [DOI] [PubMed] [Google Scholar]
- 5. Drew DA, Cao Y, Chan AT.. Aspirin and colorectal cancer: the promise of precision chemoprevention. Nat Rev Cancer. 2016;16(3):173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cuzick J, Otto F, Baron JA, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10(5):501–507. [DOI] [PubMed] [Google Scholar]
- 7. Algra AM, Rothwell PM.. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13(5):518–527. [DOI] [PubMed] [Google Scholar]
- 8. Katona BW, Weiss JM.. Chemoprevention of colorectal cancer. Gastroenterology. 2020;158(2):368–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosenberg L, Palmer JR, Zauber AG, et al. A hypothesis: nonsteroidal anti-inflammatory drugs reduce the incidence of large-bowel cancer. J Natl Cancer Inst. 1991;83(5):355–358. [DOI] [PubMed] [Google Scholar]
- 10. Giovannucci E, Rimm EB, Stampfer MJ, et al. Aspirin use and the risk for colorectal cancer and adenoma in male health professionals. Ann Intern Med. 1994;121(4):241–246. [DOI] [PubMed] [Google Scholar]
- 11. Giovannucci E, Egan KM, Hunter DJ, et al. Aspirin and the risk of colorectal cancer in women. N Engl J Med. 1995;333(10):609–614. [DOI] [PubMed] [Google Scholar]
- 12. Gann PH, Manson JE, Glynn RJ, et al. Low-dose aspirin and incidence of colorectal tumors in a randomized trial. J Natl Cancer Inst. 1993;85(15):1220–1224. [DOI] [PubMed] [Google Scholar]
- 13. Kune GA, Kune S, Watson LF.. Colorectal cancer risk, chronic illnesses, operations, and medications: case control results from the Melbourne Colorectal Cancer Study. Cancer Res. 1988;48(15):4399–4404. [PubMed] [Google Scholar]
- 14. Peleg II, Maibach HT, Brown SH, et al. Aspirin and nonsteroidal anti-inflammatory drug use and the risk of subsequent colorectal cancer. Arch Intern Med. 1994;154(4):394–399. [PubMed] [Google Scholar]
- 15. Logan RF, Little J, Hawtin PG, et al. Effect of aspirin and non-steroidal anti-inflammatory drugs on colorectal adenomas: case-control study of subjects participating in the Nottingham faecal occult blood screening programme. BMJ. 1993;307(6899):285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chubak J, Whitlock EP, Williams SB, et al. Aspirin for the prevention of cancer incidence and mortality: systematic evidence reviews for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164(12):814–825. [DOI] [PubMed] [Google Scholar]
- 17. Dehmer SP, Maciosek MV, Flottemesch TJ, et al. Aspirin for the primary prevention of cardiovascular disease and colorectal cancer: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164(12):777–786. [DOI] [PubMed] [Google Scholar]
- 18. Bibbins-Domingo K, Force U, on behalf of the U.S. Preventive Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164(12):836–845. [DOI] [PubMed] [Google Scholar]
- 19. Flossmann E, Rothwell PM, British Doctors Aspirin T, et al. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369(9573):1603–1613. [DOI] [PubMed] [Google Scholar]
- 20. Chan AT, Giovannucci EL, Meyerhardt JA, et al. Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. JAMA. 2005;294(8):914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Colditz GA, Hankinson SE.. The Nurses' Health Study: lifestyle and health among women. Nat Rev Cancer. 2005;5(5):388–396. [DOI] [PubMed] [Google Scholar]
- 22. Enright AJ. An efficient algorithm for large-scale detection of protein families. Nucleic. 2002;30(7):1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cao Y, Nishihara R, Wu K, et al. Population-wide impact of long-term use of aspirin and the risk for cancer. JAMA Oncol. 2016;2(6):762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harvard T.H. Chan School of Public Health. Health Professionals Follow-Up Study. https://sites.sph.harvard.edu/hpfs/. Accessed August 20, 2020.
- 25. Sturmer T, Glynn RJ, Lee IM, et al. Aspirin use and colorectal cancer: post-trial follow-up data from the Physicians' Health Study. Ann Intern Med. 1998;128(9):713–720. [DOI] [PubMed] [Google Scholar]
- 26. Cook NR, Lee IM, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294(1):47–55. [DOI] [PubMed] [Google Scholar]
- 27. Cook NR, Lee IM, Zhang SM, et al. Alternate-day, low-dose aspirin and cancer risk: long-term observational follow-up of a randomized trial. Ann Intern Med. 2013;159(2):77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rothwell PM, Price JF, Fowkes FG, et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379(9826):1602–1612. [DOI] [PubMed] [Google Scholar]
- 29. Rothwell PM, Fowkes FG, Belch JF, et al. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377(9759):31–41. [DOI] [PubMed] [Google Scholar]
- 30. Burn J, Sheth H, Elliott F, et al. Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: a double-blind, randomised, placebo-controlled trial. Lancet. 2020;395(10240):1855–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Movahedi M, Bishop DT, Macrae F, et al. Obesity, aspirin, and risk of colorectal cancer in carriers of hereditary colorectal cancer: a prospective investigation in the CAPP2 Study. J Clin Oncol. 2015;33(31):3591–3597. [DOI] [PubMed] [Google Scholar]
- 32. Burn J, Gerdes AM, Macrae F, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378(9809):2081–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ait OD, Dashti SG, Chau R, et al. Aspirin, ibuprofen, and the risk of colorectal cancer in lynch syndrome. J Natl Cancer Inst. 2015;107(9):djv170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Institute for Health and Care Excellence. Colorectal Cancer (NICE guideline [NG151]). https://www.nice.org.uk/guidance/ng151. Accessed August 20, 2020. [PubMed]
- 35. Chan AT, Giovannucci EL, Meyerhardt JA, et al. Aspirin dose and duration of use and risk of colorectal cancer in men. Gastroenterology. 2008;134(1):21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Friis S, Riis AH, Erichsen R, et al. Low-dose aspirin or nonsteroidal anti-inflammatory drug use and colorectal cancer risk: a population-based, case-control study. Ann Intern Med. 2015;163(5):347–355. [DOI] [PubMed] [Google Scholar]
- 37. Rothwell PM, Wilson M, Elwin CE, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376(9754):1741–1750. [DOI] [PubMed] [Google Scholar]
- 38. McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379(16):1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McNeil JJ, Gibbs P, Orchard SG, et al. Effect of aspirin on cancer incidence and mortality in older adults [published online ahead of print]. J Natl Cancer Inst. 2020;113(3):258–265.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hawk ET, Maresso KC.. The ASPREE Trial: an unanticipated stimulus for greater precision in prevention? [published online ahead of print]. J Natl Cancer Inst. 2020;113(3):221–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sandler RS, Galanko JC, Murray SC, et al. Aspirin and nonsteroidal anti-inflammatory agents and risk for colorectal adenomas. Gastroenterology. 1998;114(3):441–447. [DOI] [PubMed] [Google Scholar]
- 42. Sandler RS, Halabi S, Baron JA, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348(10):883–890. [DOI] [PubMed] [Google Scholar]
- 43. Logan RF, Grainge MJ, Shepherd VC, et al. Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology. 2008;134(1):29–38. [DOI] [PubMed] [Google Scholar]
- 44. Hull MA, Sprange K, Hepburn T, et al. Eicosapentaenoic acid and aspirin, alone and in combination, for the prevention of colorectal adenomas (seAFOod Polyp Prevention trial): a multicentre, randomised, double-blind, placebo-controlled, 2 x 2 factorial trial. Lancet. 2018;392(10164):2583–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cole BF, Logan RF, Halabi S, et al. Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials. J Natl Cancer Inst. 2009;101(4):256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Baron JA, Cole BF, Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348(10):891–899. [DOI] [PubMed] [Google Scholar]
- 47. Pommergaard HC, Burcharth J, Rosenberg J, et al. Aspirin, calcitriol, and calcium do not prevent adenoma recurrence in a randomized controlled trial. Gastroenterology. 2016;150(1):114–122.e4. [DOI] [PubMed] [Google Scholar]
- 48. Nishihara R, Lochhead P, Kuchiba A, et al. Aspirin use and risk of colorectal cancer according to BRAF mutation status. JAMA. 2013;309(24):2563–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fink SP, Yamauchi M, Nishihara R, et al. Aspirin and the risk of colorectal cancer in relation to the expression of 15-hydroxyprostaglandin dehydrogenase (HPGD). Sci Transl Med. 2014;6(233):233re2–233re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Amitay EL, Carr PR, Jansen L, et al. Association of aspirin and nonsteroidal anti-inflammatory drugs with colorectal cancer risk by molecular subtypes. J Natl Cancer Inst. 2019;111(5):475–483. [DOI] [PubMed] [Google Scholar]
- 51. Nan H, Hutter CM, Lin Y, et al. Association of aspirin and NSAID use with risk of colorectal cancer according to genetic variants. JAMA. 2015;313(11):1133–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chan AT, Ogino S, Giovannucci EL, et al. Inflammatory markers are associated with risk of colorectal cancer and chemopreventive response to anti-inflammatory drugs. Gastroenterology. 2011;140(3):799–808. quiz e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chan AT, Ogino S, Fuchs CS.. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356(21):2131–2142. [DOI] [PubMed] [Google Scholar]
- 54. Cao Y, Nishihara R, Qian ZR, et al. Regular aspirin use associates with lower risk of colorectal cancers with low numbers of tumor-infiltrating lymphocytes. Gastroenterology. 2016;151(5):879–892.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang X, Chan AT, Slattery ML, et al. Influence of smoking, body mass index, and other factors on the preventive effect of nonsteroidal anti-inflammatory drugs on colorectal cancer risk. Cancer Res. 2018;78(16):4790–4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rothwell PM, Cook NR, Gaziano JM, et al. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: analysis of individual patient data from randomised trials. Lancet. 2018;392(10145):387–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Park SY, Wilkens LR, Kolonel LN, et al. Exploring differences in the aspirin-colorectal cancer association by sex and race/ethnicity: the Multiethnic Cohort Study. Cancer Epidemiol Biomarkers Prev. 2017;26(2):162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dulai PS, Singh S, Marquez E, et al. Chemoprevention of colorectal cancer in individuals with previous colorectal neoplasia: systematic review and network meta-analysis. BMJ. 2016;355:i6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Whitlock EP, Burda BU, Williams SB, et al. Bleeding risks with aspirin use for primary prevention in adults: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164(12):826–835. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data, the statistical code, questionnaires, and technical processes are available from the corresponding author at egiovann@hsph.harvard.edu.