Figure 1.
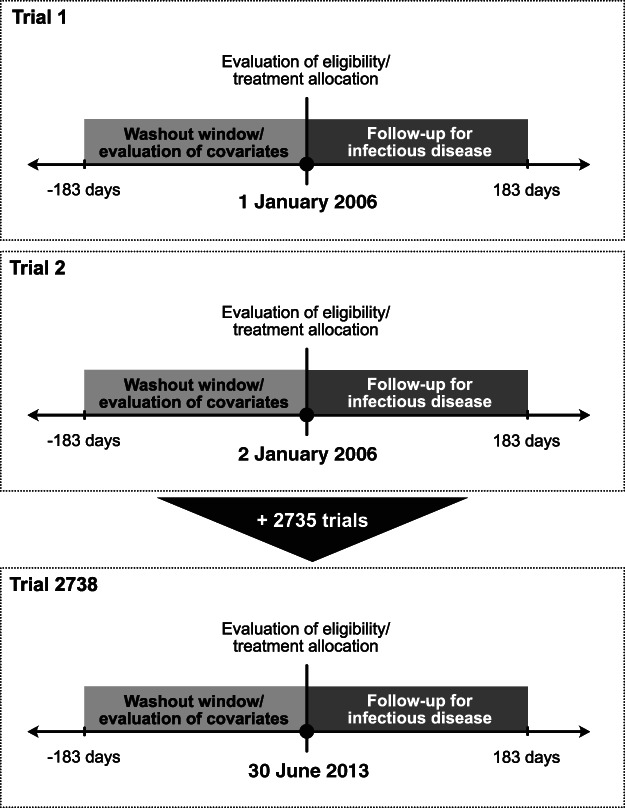
Graphical representation of the target trial emulation design including 2738 potential trial emulations nested in the Prescribed Drug Register (PDR). At each trial's initiation (i.e. time zero, depicted by a black circle, ●), eligibility criteria were evaluated (some using information collected within the previous 6 months), treatment was allocated and the 6‐month follow‐up for infectious disease started. Also, several covariates (e.g. age, sex and residential location) were evaluated at time zero some using information collected within the previous 6 months (e.g. cumulative defined daily doses of systemic corticosteroids dispensed in the PDR).
