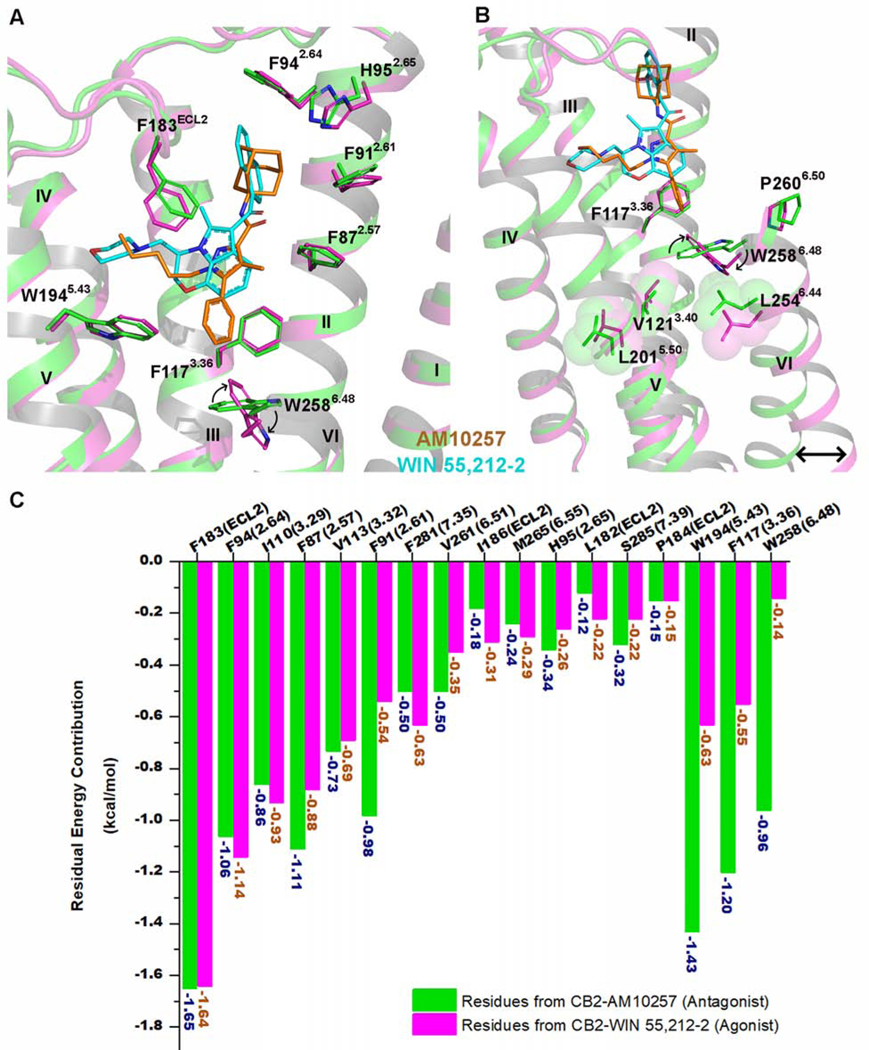
Figure 3. CB2 Activation by WIN 55,212–2.
(A) Superposition of the WIN 55,212–2 (cyan)-activated CB2 (magenta) complex with antagonist (AM10257, orange)-bound CB2 receptor (green) (PDB: 5ZTY, resolution: 2.8 Å).
(B) WIN 55,212–2 bound at the CB2 orthosteric pocket makes direct contacts with residues F1173.36 and W2586.48. The subtle rotation of F1173.36 to interact with the 2,3-dihydro-[1,4]oxazino[2,3,4-hi]indole moiety of WIN 55,212–2 allows W2586.48 to undergo a large rotation, with a consequent outward movement of the cytoplasmic end of TM6 that serves to create a cavity for G protein binding. Green cartoon: the inactive CB2 crystal structure (PDB: 5ZTY, resolution: 2.8 Å). Magenta cartoon: the active CB2 cryo-EM structure. There is relatively little rearrangement in residues V1213.40, L2015.50, and L2546.44, different from the corresponding residue Pro5.50 of β2AR and μOR, which is involved in packing interactions with Ile3.40 and Phe6.44 during the activation of these GPCRs.
(C) The energy contribution of key residues involved in the binding pockets of inactive and active CB2. Green bars: calculated energy contributions of key residues based on the inactive CB2 crystal structure (PDB: 5ZTY). Magenta bars: calculated energy contributions of key residues using the active CB2 cryo-EM structure.