Abstract
Introduction
Intermittent dosing (ID), in which periods of stimulation‐on are alternated with periods of stimulation‐off, is generally employed using 30 sec ON and 90 sec OFF intervals with burst spinal cord stimulation (SCS). The goal of this study was to evaluate the feasibility of using extended stimulation‐off periods in patients with chronic intractable pain.
Materials and Methods
This prospective, multicenter, feasibility trial evaluated the clinical efficacy of the following ID stimulation‐off times: 90, 120, 150, and 360 sec with burst waveform parameters. After a successful trial (≥50% pain relief) using ID stimulation, subjects were titrated with OFF times beginning with 360 sec. Pain, quality of life, disability, and pain catastrophizing were evaluated at one, three, and six months after permanent implant.
Results
Fifty subjects completed an SCS trial using ID stimulation settings of 30 sec ON and 90 sec OFF, with 38 (76%) receiving ≥50% pain relief. Pain scores were significantly reduced from baseline at all time points (p < 0.001). Improvements in quality of life, disability, and pain catastrophizing were aligned with pain relief outcomes; 45.8% of the subjects that completed the six‐month follow‐up visit used an OFF period of 360 seconds.
Conclusions
ID burst SCS effectively relieved pain for six months. The largest group of subjects used IDB settings of 30 sec ON and 360 sec OFF. These findings present intriguing implications for the optimal “dose” of electricity in SCS and may offer many advantages such as optimizing the therapeutic window, extending battery life, reducing recharge burden and, potentially, mitigating therapy habituation or tolerance.
Keywords: chronic pain, cycling, intermittent dosing burst, spinal cord stimulation
INTRODUCTION
Chronic pain affects up to 1.5 billion people worldwide (1). Spinal cord stimulation (SCS) is a minimally invasive and nonpharmacological treatment for chronic, intractable pain and is increasing in its application; more than 30,000 implants are completed per year in the United States (2). In a systematic meta‐analysis of the literature, SCS was reported to reduce pain, improve quality of life, reduce analgesic use, improve return to work status, and may also result in significant cost savings over time, while having a low complication rate (3).
The bulk of the existing SCS literature describes outcomes using conventional tonic stimulation, in which electrical pulses are delivered in a consistent repetitive fashion (4, 5, 6, 7). Burst stimulation (Abbott, Plano, TX, USA) delivers packets of stimulation—five pulses at 500 Hz—at a frequency of 40 Hz with repolarization occurring passively during the inter‐burst interval (8). Burst SCS has been demonstrated to be an effective treatment, with average limb pain relief of 68% after mean treatment duration of 20.5 months (9). In a large randomized controlled 12‐month trial, burst SCS provided statistically superior (albeit not clinically superior) efficacy compared with tonic SCS (10). Furthermore, 68.2% of patients preferred it over tonic stimulation, with 23.9% preferring tonic stimulation and 8% indicating no preference (10). These data were supported by a systematic review and pooled analysis including real‐world evidence for burst stimulation compared to tonic stimulation showing clinically important incremental benefits (11). In addition, recent empirical evidence employing techniques such as intraoperative electromyography (EMG) neuromonitoring (12), electroencephalography (EEG) (13) and fluorodeoxyglucose positron emission tomography (FDG‐PET) (14) suggests that burst stimulation pattern is unique among SCS waveforms. Burst SCS induces EMG responses at much lower amplitudes compared to tonic, produces a characteristic EMG signature consisting in a single large EMG spike instead of five separate ones like clustered tonic stimulation, and induces a hyper excitable state reducing thresholds for EMG activation for subsequent tonic pulses (12). Source‐localized EEG recordings demonstrated burst SCS activates the dorsal anterior cingulate and right dorsolateral prefrontal cortex more than tonic stimulation (13). Increased modulation of the dorsal anterior cingulate cortex modulation was also confirmed using FGD‐PET (14).
Significance/Rationale of Intermittent Dosing Using Burst Waveform
Cycling of stimulation, which consists of altering the amount of time stimulation is active (ON) and inactive (OFF), is a feature that has been available to SCS clinicians for years (15) and patients have always been able to activate or deactivate stimulation based on their need. Nevertheless, this tool has not been systematically studied with the goal of reducing the total amount of stimulation delivered to the spinal cord in a patient tailored fashion. Based on preclinical data (16), it was hypothesized that there would be a residual effect present for burst SCS and that, alternating ON and OFF periods of adequate duration, could provide sustained therapeutic effects while reducing the overall amount of current delivered to the spinal cord and decreasing the energy requirements of the device. This hypothesis was confirmed by the results of a randomized, cross‐over, double blinded, controlled feasibility investigation (17). Vesper et al. showed that intermittent dosing in burst SCS (IDB), using short alternating ON and OFF periods, was equivalent to continuous therapy in providing pain relief and improvement in quality of life. In their study, 25 patients who had been exclusively using burst SCS were blindly evaluated with continuously delivered stimulation as well as with two different IDB paradigms (A: 5 sec ON, 5 sec OFF, 1:1 ratio; B: 5 sec ON, 10 sec OFF, 1:2 ratio) in a randomized fashion for a period of two weeks each. Visual analog scale (VAS) scores were collected and showed no difference between the two IDB paradigms and continuous SCS (A: 0.12 ± 18.52 mm; B: −1.84 ± 23.12; ps > 0.05). Similarly, no difference was found in quality of life (EQ‐5D) between continuous stimulation and the two IDB paradigms (A: 0.03 ± 0.003; B: −0.03 ± 0.002; ps > 0.05).
Given that the optimal clinical settings for IDB are not known, the main objective of this study was to evaluate feasibility of using IDB patterns with much lower ON:OFF ratios in patients with chronic intractable pain who have never previously received SCS therapy.
MATERIALS AND METHODS
This was a prospective, open label, multicenter, feasibility trial conducted with oversight of local institutional review boards (NCT03350256). All subjects provided written informed consent prior to any study activities. Individuals diagnosed with chronic intractable back and/or leg pain, with no prior history of SCS, were eligible for the study; detailed inclusion/exclusion criteria are in Table 1. During the study, subjects completed assessments regarding their pain (standard 10‐cm VAS), quality of life (EuroQol‐5D (18)), disability (Oswestry Disability Index [ODI; (19)]), affective features of pain (Pain Catastrophizing Scale [PCS; (20)]), overall evaluation/satisfaction with treatment (patient global impression of change [PGIC]), and medication usage.
Table 1.
Demographics and Baseline Characteristics for the 50 Patients that were Trialed during the Study.
| Baseline characteristics | N (%), or mean (±CI) | |
|---|---|---|
| Gender* | Male | 19 (38.8%) |
| Female | 30 (61.2%) | |
| Age* | Years | 56.8 ± 3.95 |
| Etiology† | Failed Back Surgery Syndrome | 18 |
| Radiculopathy | 38 | |
| Degenerative disk disease | 15 | |
| Spondylosis | 15 | |
| Mild spinal stenosis | 3 | |
| Neuropathic pain | 11 | |
| Other | 11 | |
| Duration of pain‡ | Years | 9.98 ± 3.31 |
One subject missing data.
Majority of subjects had multiple indications.
Two subjects had missing data.
Subjects were implanted epidurally with bilateral percutaneous leads (Invisible Trial; Abbott, Plano, TX, USA). Intraoperative paresthesia mapping was performed with the goal of positioning the leads to obtain coverage of painful anatomies. Subjects then completed a trial period of three to sevendays using an external trial stimulator. During the trial, all subjects used paresthesia‐free burst stimulation with amplitude set to 60% of the perception threshold and with an ID setting of 30 sec ON and 90 sec OFF (1:3 ratio). Subjects who received at least 50% back and/or leg pain relief at the end of the trial, expressed interest in a permanent implant and were deemed successful by the treating physician received a permanent implant.
Following permanent implantation, subjects underwent a titration of their ID settings. Titration started shortly after activation and was performed only once. Paresthesia‐free BurstDR stimulation was initiated for two to three days with a small electrical dose via a low ID setting (30 sec ON and 360 sec OFF; a 1:12 ratio). If the subject reported that this resulted in pain control equal to or better than during the trial, that ID setting was continued as the subject's individual dosed treatment. Otherwise, the electrical dose was increased by changing to the next‐in‐line ID setting with a shorter OFF time; see Fig. 1) and this was evaluated for another two to three days. This continued, if needed, in a stepwise fashion through four other options until reaching the same ID setting as used during the trial period. Once a subject moved to an ID setting with a shorter OFF time, they did not return to previously tested ones. Thus, the ID settings tested, in order, were 30 sec ON and 360 sec OFF (1:12), 30 sec ON and 240 sec OFF (1:8), 30 sec ON and 150 sec OFF (1:5), 30 sec ON and 120 sec OFF (1:4), and 30 sec ON and 90 sec OFF (1:3). In this way, subjects self‐selected the lowest dose that achieved pain control similar to that of the trial period. The use of the 30 sec ON and 90 sec OFF ID setting, which was identical to trial, ensured that unsatisfactory results observed after the trial could not be ascribed to the use of ID itself. Subjects agreed to not to increase pain medications until the three‐month follow‐up visit. ID settings were captured at the six‐month follow‐up visit.
Figure 1.
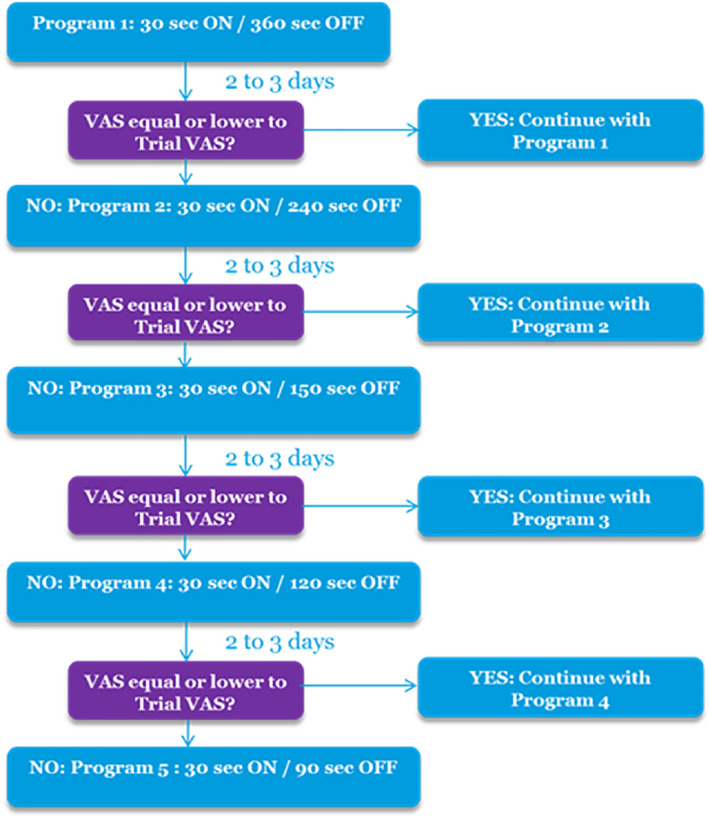
Schematic representation of post‐trial IDB titration process. [Color figure can be viewed at wileyonlinelibrary.com]
After one, three, and six months of treatment, the baseline assessments were repeated, along with overall evaluation/satisfaction with treatment (PGIC). Any subject whose VAS score at the three‐ or six‐month follow‐up was ≥20 mm higher than that at the one‐month follow‐up was identified as having experienced reduction of efficacy (21). Adverse events occurring at any point during the study were captured.
Data are reported as means, medians, 95% confidence intervals (CI), or percentages. Since normality of data was not assumed, a non‐parametric repeated measures ANOVA (Friedman's test) was conducted to determine main effects using the Cochran–Mantel–Haenszel Statistics (Q). Wilcoxon signed‐rank test comparisons were performed to determine specific differences between visits. A retrospective post‐hoc exploratory analysis was conducted to compare the group of subjects using an OFF period of 360 sec with the subjects using the three shortest OFF periods (150, 120, and 90 sec, which were combined in a single group in order to increase sample size). Only the three shortest intervals were selected as they are less than half of the duration of the longer OFF period thus allowing a marked difference in OFF duration between the two groups. These differences were determined by a non‐parametric Mann–Whitney test. All tests were conducted using SAS 9.4. Significance was set at p = 0.05.
RESULTS
The study screened 60 patients. Out of the 60 patients screened, 10 subjects were withdrawn prior to the trial phase for various reasons, including: inclusion/exclusion violations (n = 4), subjects recommended to receive other therapies (n = 2), protocol violation (n = 1), noncompliance (n = 1), denial of insurance (n = 1), and an adverse event (n = 1). Thus, 50 subjects (average age of 56.8 ± 3.95 years, reporting having chronic pain for an average of 9.98 ± 3.31 years, see Table 1), received a trial system and continued in the study. Thirty‐eight subjects (76%) reported at least a 50% reduction in either their back and/or leg pain and were deemed eligible for a permanent implant. Of these, 35 subjects received permanent implant as three subjects elected not to proceed to implant. Additionally, 11 subjects were withdrawn during the follow‐up phase for various reasons, including: patient chose to withdraw (3), protocol violation (4), nonstudy related adverse event (1), noncompliance (1), subject moving out of state (1), and lost to follow‐up (1).
IDBSCS Settings Usage
After permanent implantation, subjects sequentially tested the IDB patterns, starting with the option with the longest stimulation‐off duration (360 sec OFF). At the six‐month follow‐up, the percentage of subjects using each stimulation ON:OFF ratio was as follows: 45.8% of patients were using an ON:OFF ratio of 1:12, 12.5% an ON:OFF ratio of 1:8, 12.5% an ON:OFF ratio of 1:5, 12.5% an ON:OFF ratio of 1:4, and 16.7% an ON:OFF ratio of 1:3 (see Fig. 2a).
Figure 2.
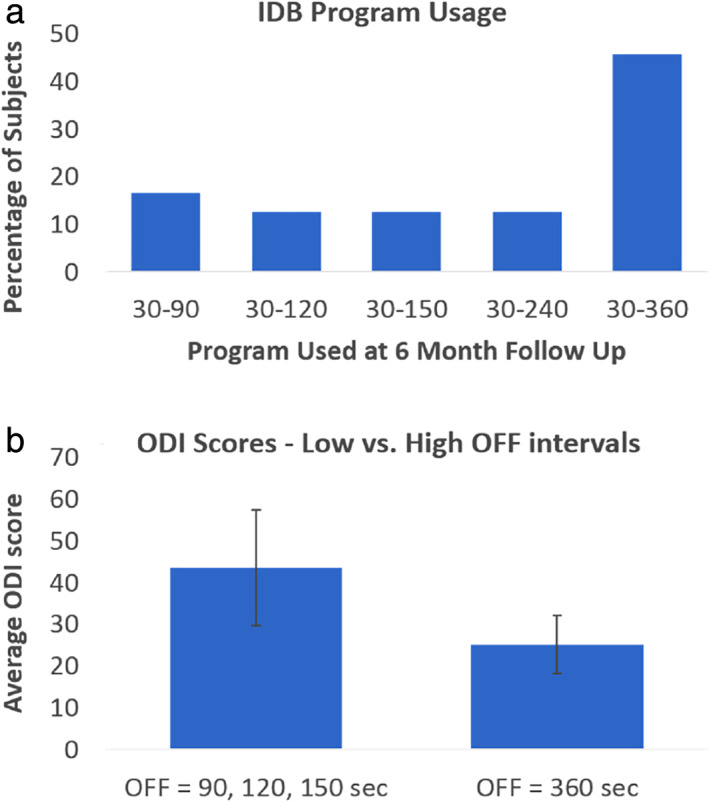
(a): IDB program usage at the six‐month follow‐up visit. (b) Average ODI scores at the six‐month follow‐up of subjects who used the three shorter OFF periods and subjects who used the longer OFF periods (150, 120, and 90 sec OFF). Error bars represents 95% confidence intervals. Statistical differences were observed between the two OFF groups using a Mann–Whitney test. [Color figure can be viewed at wileyonlinelibrary.com]
Retrospective analysis showed that there was no statistical difference in VAS, EQ‐5D, or PCS at six months between the group of subjects using an OFF period of 360 sec and the subjects using the three shortest OFF periods. However, subjects using the 360 sec OFF time had a significantly lower mean ODI score (25.3 ± 7.3; median = 24.0) than the other group (43.6 ± 14.6; median = 49.0) p = 0.04, Z = 1.97) at six months (see Fig. 2b). The ODI scores were not statistically different at baseline between the two groups.
Pain Intensity Assessment
The mean (CI) baseline overall VAS score was 77.2 ± 3.6 mm (median = 80.0 mm). Average overall VAS score improved to 30.8 ± 7.4 mm (median = 24.0 mm) at the end of the trial (n = 47), 33.8 ± 7.9 mm (median = 31.5 mm) at the one‐month follow up (n = 28), 35.6 ± 10.9 mm (median = 30.0 mm) at the three‐month follow‐up (n = 25) and 37.1 ± 10.0 mm (median = 42.0 mm) at the six‐month follow‐up (n = 24) (see Fig. 3a). Overall VAS scores were significantly different from baseline at all later time points (p < 0.001, Q [4] = 76.89) but did not differ amongst themselves. Similarly, average back pain VAS improved from 73.7 ± 4.4 mm (median = 74.0 mm) at baseline (n = 50) to 29.8 ± 7.8 mm (median = 20.0 mm) at the end of trial (n = 47), 31.2 ± 9.4 mm (median = 28.0 mm) at the one‐month follow‐up (n = 28), 36.8 ± 11.6 mm (median = 30.0 mm) at the three‐month follow‐up (n = 25) and 39.6 ± 10.8 mm (median = 42.5 mm) at the six‐month follow‐up (n = 24) (see Fig. 3b). Back VAS scores were significantly different from baseline at all later time points (p < 0.001, Q [4] = 63.85) but did not differ amongst themselves. Average leg pain VAS improved from 72.1 ± 6.1 mm (median = 76.0 mm) at baseline (n = 50) to 31.8 ± 8.2 mm (median = 23.0 mm) at the end of trial (n = 47), 26.1 ± 9.2 mm (median = 21.0 mm) at the one‐month follow‐up (n = 28), 29.0 ± 11.7 mm (median = 15.0 mm) at the three‐month follow‐up (n = 25) and 29.5 ± 9.8 mm (median = 19.0 mm) at the six‐month follow‐up (n = 24) (see Fig. 3c). Leg VAS scores were significantly different from baseline at all later time points (p < 0.001, Q [4] = 58.86) but did not differ among themselves. Ninety‐one percent of subjects maintained efficacy for overall and back pain. All subjects (100%) maintained efficacy for leg pain.
Figure 3.
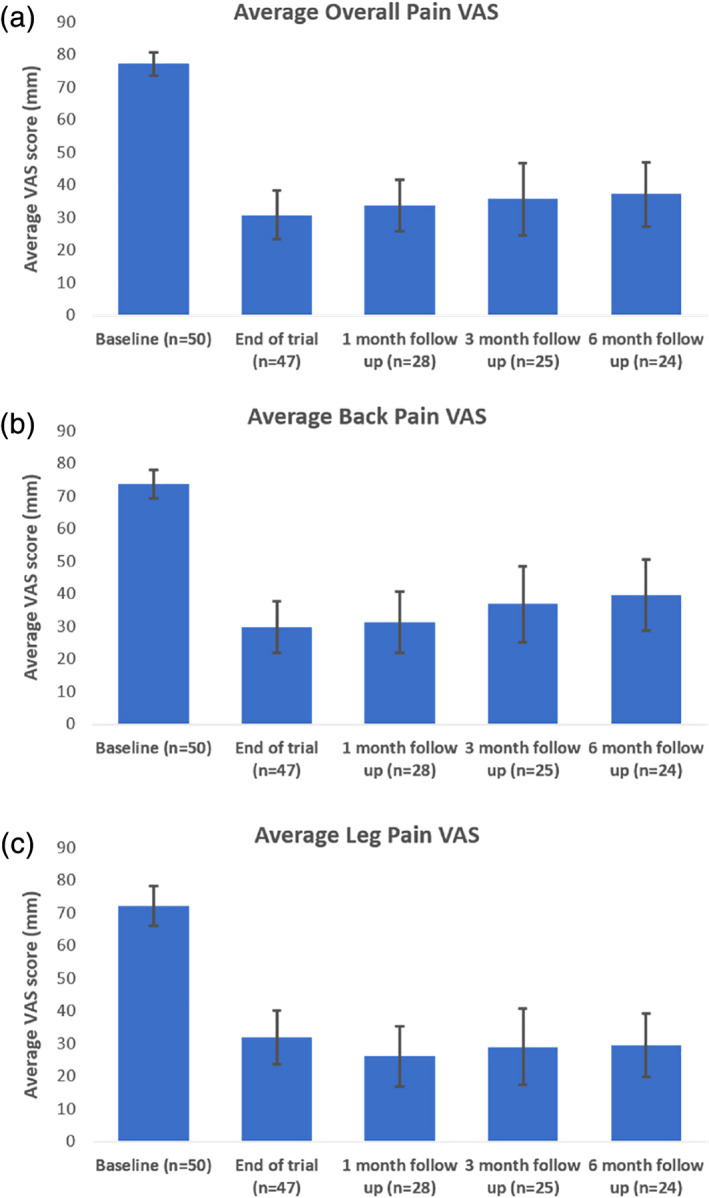
Effect of titrated IDB SCS on pain. Overall (a), back (b), and leg (c) average pain at baseline and at follow‐up visits (p < 0.001). Error bars represents 95% confidence intervals. Statistical differences were observed between baseline and all other follow‐up visits using Friedman's test. [Color figure can be viewed at wileyonlinelibrary.com]
Quality of Life and Functionality Assessments
The mean EQ‐5D index improved significantly from 0.51 ± 0.04 (median = 0.53; n = 49) at baseline to 0.69 ± 0.06 (median = 0.78; n = 47) at the end of trial, 0.69 ± 0.06 at the one‐month follow‐up (median = 0.74; n = 28), 0.67 ± 0.10 (median = 0.79) at the three‐month follow‐up (n = 26), and 0.69 ± 0.08 (median = 0.74) at the six‐month follow‐up (n = 24). Values significantly differed from baseline at all later time points (p < 0.001, Q [4] = 34.54) but did not differ amongst themselves (see Fig. 4a).
Figure 4.
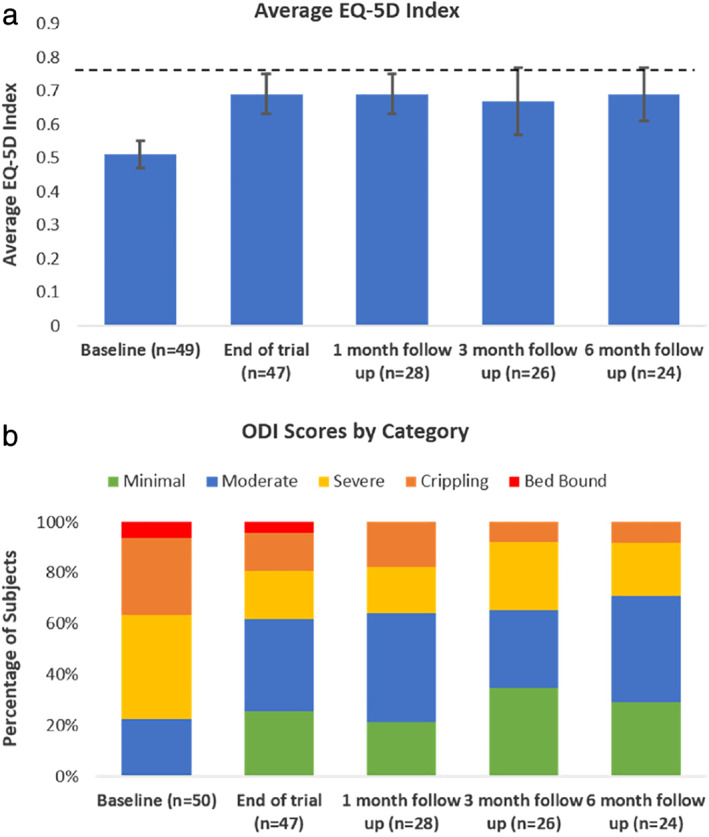
Effect of titrated IDB SCS on subjects' functionality. (a) Average EQ‐5D scores at baseline, trial and at follow‐up visits (p < 0.001); dashed line represents U.S. population norm for age group 55–64 years (0.776 (22)), error bars represents 95% confidence intervals. Statistical differences were observed between baseline and all other follow‐up visits using Friedman's test. (b) Percentage of subjects in each of the Oswestry Disability Index categories at baseline, trial, and at follow‐up visits. [Color figure can be viewed at wileyonlinelibrary.com]
Back pain‐related disability as evaluated on ODI decreased from 54.3 ± 4.9 (median = 56.0; n = 49) at baseline to 36.0 ± 6.88 (median = 32.0; n = 47) at the end of trial, 37.7 ± 7.72 (median = 37.8) at the one‐month follow‐up (n = 28), 31.5 ± 8.86 (median = 22.6) at the three‐month follow‐up (n = 26) and 33.3 ± 8.12 (median = 33.0) at the six‐month follow‐up (n = 24). Values significantly differed from baseline at all later time points (p < 0.001, Q [4] = 27.70) but did not differ amongst themselves. Reduction in severity of disability was observed from baseline throughout the follow up period (see Fig. 4b).
Psychometric Assessment and Satisfaction
Pain catastrophizing as evaluated using the PCS questionnaire decreased from 24.5 ± 3.9 (median = 24.0; n = 49) at baseline to 14.6 ± 4.0 (median = 10.0; n = 47) at the end of trial, 10.1 ± 3.4 (median = 7.5) at the one‐month follow‐up (n = 28), 9.6 ± 4.7 (median = 4.0) at the three‐month follow‐up (n = 26) and 9.5 ± 4.6 (median = 6.0) at the six‐month follow‐up (n = 23). Values differed significantly from baseline at all later time points (p < 0.001, Q [4] = 35.03) but did not differ among themselves (see Fig. 5a). Similarly, mean pain catastrophizing sub‐scores (rumination, magnification, and helplessness) were significantly different from baseline at all‐time points (p < 0.001; see Fig. 5b).
Figure 5.
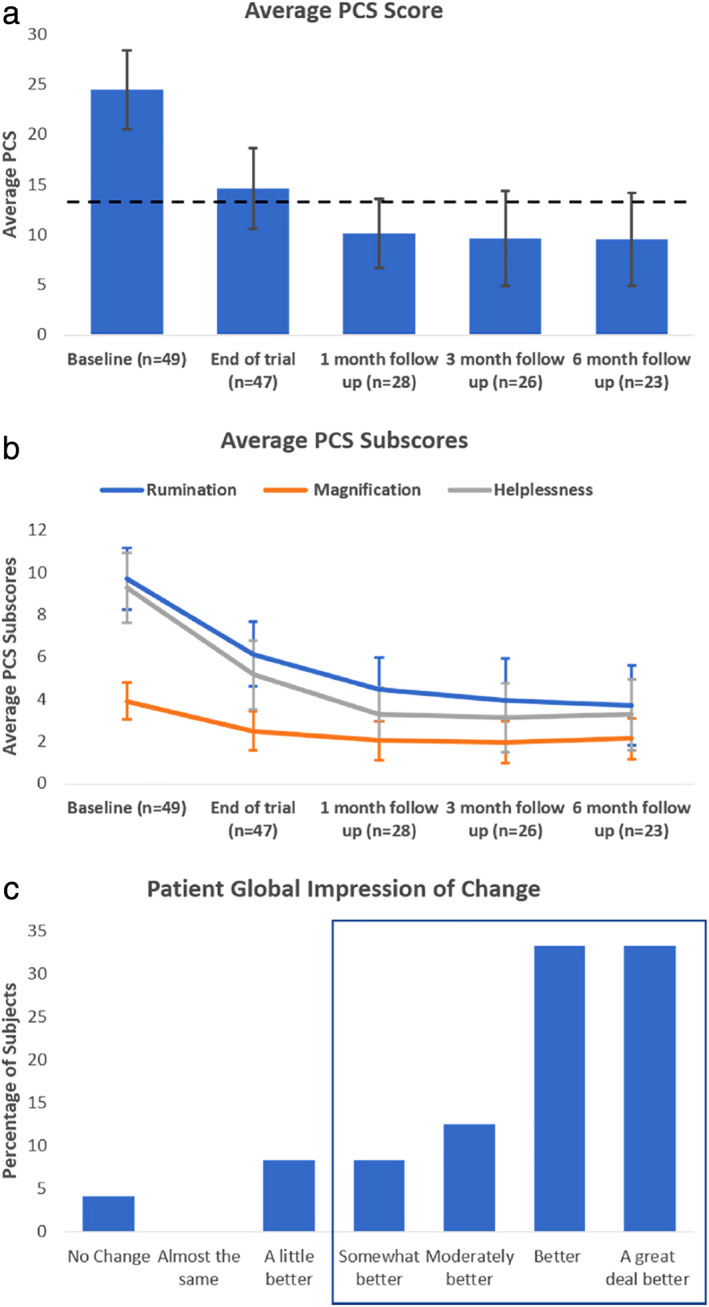
Effect of titrated IDB SCS on subjects' perception of pain and impression of change. (a) Average Pain Catastrophizing Scale scores (p < 0.001); dashed line represents nonpain population norm (13.9 (20)), error bars represents 95% confidence intervals. Statistical differences were observed between baseline and all other follow‐up visits using Friedman's test. (b) Average Pain Catastrophizing Scale subcomponents, error bars represent 95% confidence intervals. (c) Patient General Impression of Change at the six‐month follow‐up visit; blue box contains the categories that reported “somewhat better” or more marked impression of change. [Color figure can be viewed at wileyonlinelibrary.com]
The impact of the treatment on change in subjects' life was assessed using PGIC scale. When asked to “Rate the overall change in activity limitations, symptoms, emotions, and overall quality of life since starting SCS therapy”, 87.5% of subjects reported they were “somewhat better,” “moderately better,” “better,” or “a great deal better” at the six month follow‐up (n = 24) (see Fig. 5c).
Adverse Events
A total of eight adverse events were reported in the study, of which, four were considered device‐related. These were lead migrations during trial (n = 3) and pocket heating (n = 1), which was resolved after turning the stimulator off for three days. There were two serious adverse events that were not study related (fractured vertebrae, abdominal pain). There were no unexpected device‐related adverse events during this study.
DISCUSSION
Our results demonstrate that IDB SCS is safe and efficacious in subjects with chronic back and/or leg pain and can be successfully used with OFF periods extending up to 360 sec. Subjects, effectively self‐selecting the length of the OFF period, received on average > 50% pain relief between baseline and six months. At the six‐month follow‐up, 45.8% of the subjects used the 360 sec OFF period (a 1:12 ON:OFF ratio), while the remainder of the subjects distributed evenly over the shorter intervals. At the same time, subjects' quality of life and functionality improved, as indicated by a significant increase in reported quality of life and a significant decrease in reported disability. Additionally, subjects reported a significant reduction of pain catastrophizing and 87.5% had positive satisfaction in the patient general impression of change at six months. These results suggest that equivalent clinical outcomes can be obtained using low ON:OFF ratios.
Conceptual Similarity to Pharmaceutical Dosage
Given our lack of detailed understanding on potential electrical effects of heating on the spinal cord, with increasing energy equating to increased heat dissipation on the spinal cord, and induction of neural accommodation/tolerance in relation to dose of energy delivered, we felt that this provided the rationale for the aforementioned clinical design strategy with a focus on dosing and titration.
In addition, to explore electrical dosing, concepts such as pharmacokinetics and pharmacodynamics that are well‐understood concepts in the field of medicine become extremely relevant. While their extension to the field of neuromodulation is not straightforward, and the study did not evaluate or show evidence of dose response curves like pharmacological studies, both concepts can serve as guides to better understanding the effects of electrical stimulation on the nervous system and advance the approach taken to delivery of neuromodulation toward a more patient centric approach. Pharmacokinetics is defined as the study of the time course of drug adsorption, distribution, metabolism and excretion. In the neuromodulation field, these concepts can be extrapolated to the basic principles of how electrical stimuli are delivered (absorption), spread (distribution) and are dissipated through heat generation (metabolism and excretion) within the simulated area. Pharmacodynamics refers to the relationship between drug concentration and the physiological effect, including the time course. In the neuromodulation field, these concepts can be extended to amount of current delivered over a unit of time (concentration), the produced alteration of neuronal activity (physiological effect) and persistence in time of such physiological effects (time course).
Based on pharmacokinetic and pharmacodynamic principles, dosages are generated to deliver the ideal amount of active compound to the intended target and to ensure the concentration of such agent is within the desired therapeutic window (above the lowest concentration capable of producing the desired physiological effects and below the concentration at which side effects will manifest). Similar concepts can be useful also in the neuromodulation field where dosage is represented by the specific combination of stimulation amplitude, pulse width, stimulation frequency, impedance, and duty cycle. Likewise, therapeutic window can be identified as the difference between the combination of stimulation parameters (“dose”) providing the required physiological outcome with the minimum amount of electrical current delivered to the spine and the parameters that first produce undesired stimulation effects. While delivery of electrical stimulation to the target is immediate, the effects produced can have different time constants. The concept of therapeutic range, latency, and persistency of effects is not novel concepts in SCS. Indeed, the role of stimulation parameters and their role in electrical charge delivered have been previously discussed (23). Likewise, Shealy et al. in their seminal paper observed a short carry over effect of SCS in cats receiving electrical stimulation of the dorsal column and noxious stimulation but also suggested that continuous stimulation is necessary in patients with continuous pain (24). Other authors have instead reported that the pain‐relieving effect of SCS can last after stimulation has been discontinued albeit with significant differences between patients (15, 25). Latency and persistency of effects are a well‐known fact in deep brain stimulation, where different conditions and even different symptoms of the same condition require different amounts of time to re‐emerge after stimulation is discontinued. In Parkinson's disease, tremor returns in seconds, bradykinesia can take minutes while axial symptoms even longer (26). This phenomenon is driven by the dynamics of the nervous system and can be potentially elegantly exploited to use intermittent stimulation as an additional parameter to more precisely titrate electrical stimulation in order to provide the desired outcome, while minimizing the overall amount of electrical stimulation. Future sham‐controlled studies, with a shorter time resolution, will be necessary to fully characterize SCS parameters in a fashion analogous to pharmacokinetic and pharmacodynamic characterization of pharmacological compounds.
Primary Cell Battery Life and Recharge Burden
Additionally, use of these new lower dose stimulation parameters has great potential to prolong the battery life of a nonrechargeable primary cell IPG. The average time between battery‐replacement surgeries for conventional SCS systems has been estimated to be three to fiveyears (27, 28, 29) and while no data on battery consumption were collected during this study, it is reasonable to expect that IDB using a low ON:OFF ratio may decrease the frequency of battery replacements. Rechargeable IPGs afford longer times between replacement but were found to have higher likelihood of explant due to lack of therapeutic efficacy (30). The obligation of frequent recharging has been identified as a negative attribute by some SCS patients (31) and could be contributing to the higher explant rate. The reduction in current delivered to the spinal column using IDB will also improve convenience for these patients by decreasing the frequency of recharging sessions and the associated burden.
Burst Waveform Tends to Allow for Unique Intermittent Dosing Burst Programming
Given the variety of waveforms currently clinically available, the question of whether the effects of ID are unique to burst stimulation waveform versus clustered tonic stimulation remains to be further explored. However, as this waveform is most natural (32) with lower per pulse charge delivered (33), there is good suggestion that this waveform lends itself to the concept of lowest effective dose based on absolute energy dispersion. This extrapolation has been organized in the DBS literature as the total electrical energy delivered (TEED) equation that aims to quantify the energy in terms of pulse width, amplitude squared, frequency over impedance with duty cycle playing a role.
CONCLUSIONS
The results of this study show that IDB SCS can provide effective pain relief, increase quality of life, reduce disability, and decrease pain catastrophizing even when low ON:OFF ID ratios (1:12, for a total of 8% on time) are used. These results suggest that intermittent stimulation can be titrated to provide optimal pain relief while delivering the lowest possible dose of electricity to the spinal cord (although no dose response curve was observed in this study). This may help extend device battery life and potentially reduce or delay therapy habituation. Although preliminary, the results from this study indicate that titration of IDB can be used to provide successful pain relief while reducing the amount of electrical current delivered to the spinal cord.
Authorship Statement
All authors made a substantial contribution to the study's concept and design. Drs. Deer, Patterson, Mehta, Pope and Baksh have conducted the study, including patient recruitment and data collection. Dr. Agnesi and Mr. Raza have performed data analysis and prepared the manuscript with important intellectual contribution from Dr. Deer, Dr. Patterson, Dr. Mehta, Dr. Pope, Dr. Baksh, and Dr. Chakravarthy. All authors have approved the final version of the manuscript.
COMMENTS
The message of this paper is that in many cases it appears possible to achieve similar analgesia with a stimulator system running less than 10% of the time as with continuous operation. This is a remarkable result. If the effect endures, it suggests that the lifetime of primary cell pulse generators can be very substantially extended. An important question now is whether cycled therapy retains its efficacy over time to the same degree as continuous SCS. Longer term follow up studies are therefore critical. In addition to the potential implications for battery life, the study raises the interesting question of whether the reduction in on‐time might reduce any tendency to the development of tolerance. If that is the case, one might expect cycled SCS to hold its efficacy better than continuous SCS. Again, long term follow up will be needed to answer this question.
James FitzGerald, MA, BM, BCh, PhD
Oxford, United Kingdom
***
The study is very interesting, confirming the hypothesis of Vesper et al. that lower energy dosing could be as effective as higher ones, and furthermore demonstrating that it can even be more effective. Apart from the data of the study, the considerations on device "economics", in terms of battery consumption and patient comfort, on plasticity and habituation, and especially on kinetics and dynamics of neural electrical stimulation, are very interesting and a very good topic for further discussion.
Laura DeMartini, MD
Pavia, Italy
Acknowledgement
The authors thank Allison Foster, PhD, an independent medical writer, for her intellectual contribution to the drafting of the manuscript.
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to http://www.wiley.com/WileyCDA/Section/id-301854.html
Source(s) of Financial Support: This study was funded by Abbott.
[The copyright line for this article was changed on 08 April 2020 after original online publication.]
Conflict of Interest: Dr. Deer is a consultant for Axonics, Abbott, Nalu, Saluda, Stimgenics, Flowonix, Vertos, Vertiflex, Spinethera, Jazz. He is a minor equity holder for Saluda, Nalu, Spinethera, Stimgenics, Vertiflex, Vertos, and Bioness. Dr. Patterson is a consultant, investigator, advisory board member, and proctor for Vertiflex; consultant and speaker for Amgen; consultant, investigator, proctor, and speaker for Abbott Medical/St Jude Medical; consultant, proctor, and speaker for Allergan; and consultant, advisory board member, and proctor for CornerLoc. Dr. Baksh is a consultant for Abbott and Flowonix Medical. Dr. Jason Pope is a consultant for Abbott, Flowonix, Jazz Pharmaceuticals, Medtronic, Nevro and Saluda. Dr. Mehta is a consultant and speaker for Abbott and Vertiflex and an advisory board member for Vertiflex. Mr. Adil Raza and Dr. Filippo Agnesi are employees with Abbott. Dr. Chakravarthy is consultant to Abbott, Bioness, SPR Therapeutics, Medincell, and founder of NanoAxis, Newrom Biomedical, and Douleur Therapeutics. Drs. Deer, Patterson, Mehta, and Chakravarthy are members of an advisory committee for Abbott.
REFERENCES
- 1. Borsook D. A future without chronic pain: neuroscience and clinical research. Cerebrum 2012;2012:7–23. [PMC free article] [PubMed] [Google Scholar]
- 2. Prager J. Estimates of annual spinal cord stimulator implant rises in the United States. Neuromodulation. 2010;13:68–69. [DOI] [PubMed] [Google Scholar]
- 3. Taylor RS. Spinal cord stimulation in complex regional pain syndrome and refractory neuropathic back and leg pain/failed fack surgery syndrome: results of a systematic review and meta‐analysis. J Pain Sympt Manage 2006;31:S13–S19. [DOI] [PubMed] [Google Scholar]
- 4. Nissen M, Ikaheimo TM, Huttunen J, Leinonen V, von und zu Fraunberg M. Long‐term outcome of spinal cord stimulation in failed back surgery syndrome: 20 years of experience with 224 consecutive patients. Neurosurgery 2019;84:1011–1018. [DOI] [PubMed] [Google Scholar]
- 5. Maher DP, Martins YC, Doshi T et al. Neuropathic pain medication use does not alter outcomes of spinal cord stimulation for lower extremity pain. Neuromodulation 2018;21:106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kumar K, Taylor RS, Jacques L et al. The effects of spinal cord stimulation in chronic pain are sustained: a 24‐month follow‐up of the prospective randomized controlled multicenter trial of the effectivness of spinal cord stimulation. Neurosurgery 2008;63:762–770. [DOI] [PubMed] [Google Scholar]
- 7. Taylor RS, Desai MJ, Rigoard P, Taylor RJ. Predictors of pain relief following spinal cord stimulation in chronic back and leg pain and failed back surgery syndrome: a systematic review and meta‐regression analysis. Pain Pract 2014;14:489–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Ridder D, Vancamp T, Vanneste S. Fundamentals of burst stimulation of the spinal cord and brain. In: Krames ES, Peckham PH, Rezai AR, editors. Neuromodulation: comprehensive textbook of principles, technologies, and therapies. 2nd ed. London, UK: Academic Press; 2018. [Google Scholar]
- 9. De Ridder D, Vanneste S, Plazier M, Van Der Loo E, Menovsky T. Burst spinal cord stimulation: toward paresthesia‐free pain suppression. Neurosurgery 2010;66:986–990. [DOI] [PubMed] [Google Scholar]
- 10. Deer T, Slavin KV, Amirdelfan K et al. Success using neuromodulation with BURST (SUNBURST) study: results from a prospective, randomized controlled trial using a novel burst waveform. Neuromodulation 2018;21:56–66. [DOI] [PubMed] [Google Scholar]
- 11. Chakravarthy K, Malayil R, Kirketeig T, Deer T. Burst spinal cord stimulation: a systematic review and pooled analysis of real‐world evidence and outcomes data. Pain Med 2019;20:S23–S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Falowski SM. An observational case series of spinal cord stimulation waveforms visualized on intraoperative neuromonitoring. Neuromodulation 2019;22:219–228. [DOI] [PubMed] [Google Scholar]
- 13. De Ridder D, Plazier M, Kamerling N, Menovsky T, Vanneste S. Burst spinal cord stimulation for limb and back pain. World Neurosurg 2013;5:642–649. [DOI] [PubMed] [Google Scholar]
- 14. Yearwood T, De Ridder D, Yoo HB et al. Comparison of neural activity in chronic pain patients during tonic and burst spinal cord stimulation using fluorodeoxyglucose positron emission tomography. Neuromodulation 2020;23:56–63. [DOI] [PubMed] [Google Scholar]
- 15. Wolter T, Winkelmuller M. Continuous versus intermittent spinal cord stimulation: an analysis of factors influencing clinical efficacy. Neuromodulation 2012;15:13–19. [DOI] [PubMed] [Google Scholar]
- 16. Weisshaar CL, Kent AR, Venkatesan L, Winkelstein BA. Comparison of burst SCS paradigms on acute spinal neuronal activity in a rat model of painful radiculopathy. San Diego, CA: Paper presented at American Society of Regional Anesthesia and Pain Medicine, 2016.
- 17. Vesper J, Slotty P, Schu S et al. Burst SCS microdosing is as efficacious as standard burst SCS in treating chronic back and leg pain: results from a randomized controlled trial. Neuromodulation. 2019;22:190–193. [DOI] [PubMed] [Google Scholar]
- 18. Szende A, Oppe M, Devlin N. EQ‐5D value sets: inventory, comparative review and user guide (EuroQol group monographs). Dordrecht, The Netherlands: Springer, 2010. [Google Scholar]
- 19. Fairbank JC, Couper J, Davies JB. The Oswestry low back pain disability questionnaire. Physiotherapy 1980;66:271–273. [PubMed] [Google Scholar]
- 20. Osman A, Barrios FX, Gutierrez PM, Kopper BA, Merrifield T, Grittmann L. The Pain Catastrophizing Scale: further psychometric evaluation with adult samples. J Behav Med 2000;23:351–365. [DOI] [PubMed] [Google Scholar]
- 21. Aiudi CM, Dunn RY, Burns SM et al. Loss of efficacy to spinal cord stimulator therapy: clinical evidence and possible causes. Pain Physician 2017;20:E1073–E1080. [PubMed] [Google Scholar]
- 22. Szende A, Janssen B, Cabases J, editors. Self‐reported population health: an international perspective based on EQ‐5D. Dordrecht/Heidelberg/New York/London: Springer; 2014. [PubMed] [Google Scholar]
- 23. Miller JP, Eldabe S, Buchser E, Johanek LM, Guan Y, Linderoth B. Parameters of spinal cord stimulation and their role in electrical charge delivery: a review. Neuromodulation 2016;19:373–384. [DOI] [PubMed] [Google Scholar]
- 24. Shealy CN, Mortimer JT, Hagfors NR. Dorsal column electroanalgesia. J Neurosurg 1970;32:560–564. [DOI] [PubMed] [Google Scholar]
- 25. North RB, Fischell TA, Long DM. Chronic stimulation via percutaneously inserted epidural electrodes. Neurosurgery 1977;1:215–218. [DOI] [PubMed] [Google Scholar]
- 26. Agnesi F, Johnson MD, Vitek JL. Deep brain stimulation: how does it work? Handb Clin Neurol 2013;116:39–54. [DOI] [PubMed] [Google Scholar]
- 27. Kumar K, Malik S, Demeria D. Treatment of chronic pain with spinal cord stimulation versus alternative therapies: cost‐effectiveness analysis. Neurosurgery 2002;51:106–116. [DOI] [PubMed] [Google Scholar]
- 28. Hornberger J, Kumar K, Verhulst E, Clark MA, Hernandez J. Rechargeable spinal cord stimulation versus nonrechargeable system for patients with failed back surgery syndrome: a cost‐consequences analysis. Clin J Pain 2008;24:244–252. [DOI] [PubMed] [Google Scholar]
- 29. Taylor RS, Ryan J, O'Donnell R, Eldabe S, Kumar K, North RB. The cost‐effectiveness of spinal cord stimulation in the treatment of failed back surgery syndrome. Clin J Pain 2010;26:463–469. [DOI] [PubMed] [Google Scholar]
- 30. Van Buyten JP, Wille F, Smet I et al. Therapy‐related explants after spinal cord stimulation: results of an international retrospective chart review study. Neuromodulation. 2017;20:642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lam CK, Rosenow JM. Patient perspectives on the efficacy and ergonomics of rechargeable spinal cord stimulators. Neuromodulation. 2010;13:218–223. [DOI] [PubMed] [Google Scholar]
- 32. De Ridder D, Vanneste S, Plazier M, Vancamp T. Mimicking the brain: evaluation of St. Jude Medical's prodigy chronic pain system with burst technology. Exp Rev Med Dev 2015;12:143–150. [DOI] [PubMed] [Google Scholar]
- 33. de Vos CC, Bom MJ, Vanneste S, Lenders MWPM, de Ridder D. Burst spinal cord stimulation evaluated in patients with failed back surgery syndrome and painful diabetic neuropathy. Neuromodulation. 2013;17:152–159. [DOI] [PubMed] [Google Scholar]


