FIGURE 1.
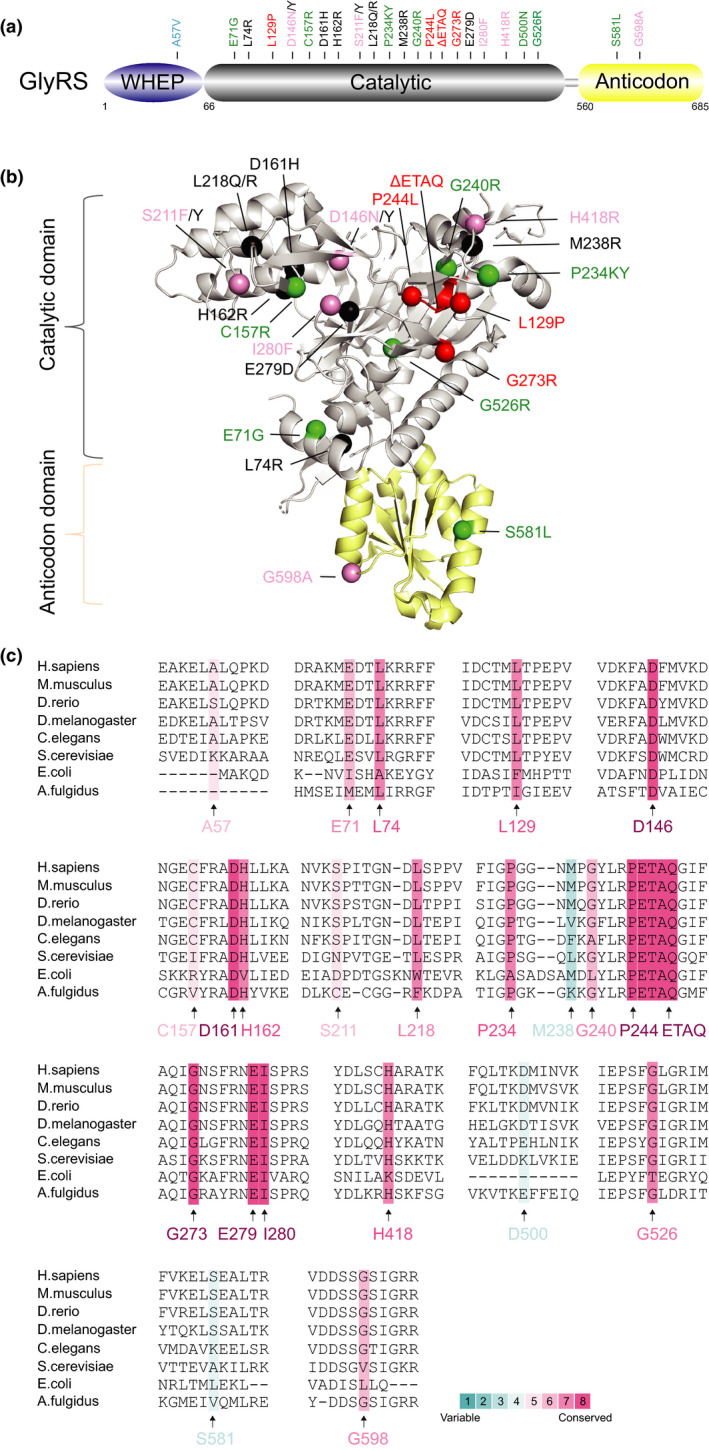
Distribution and conservation of CMT‐associated variant sites in human GlyRS. (a) Functional domains of GlyRS including a WHEP domain (in purple), a catalytic domain (in grey) and an anticodon domain (in yellow). (b) The crystal structure of human GlyRS (PDB entry 2PME). CMT variant sites in either schematic diagram (a) or crystal structure (b) are indicated with different colors based on enzymatic activity of each variant. CMT variants with WT‐like enzymatic activity (fully active) are colored in green; variants with the activity ≥1/2 are labeled in blue; variants with the activity <1/2 are indicated in pink; variants with no activity (inactive) are colored in red; variants with activity undetermined are indicated in black. The priority of enzymatic activity displayed here is ranked based on aminoacylation assays in patient sample > animal models > in vitro using purified human enzyme > yeast orthologs. (c) Evolutionary conservation analysis of CMT‐linked GlyRS across archaea, bacteria and eukaryotes. Sequence alignment of each GlyRS variant site is indicated by the color intensity, with blue representing variable and red representing conserved
