Abstract
Aims
Whether diabetes increases venous thromboembolism (VTE) is unclear. Any greater risk may relate to insulin resistance, but many studies did not differentiate between type 1 diabetes and type 2 diabetes for VTE risk.
Methods
Retrospective cohort study of the Royal College of General Practitioners Research and Surveillance Centre, comprising over 530 primary care practices. We determined whether type 1 diabetes and/or type 2 diabetes are independent risk factors for VTE. The index date was 1 January 2009, individuals were followed to 31 December 2018, or censoring. Cox proportional hazard regression analysis was used to investigate the risk of VTE in people with type 1 diabetes and type 2 diabetes relative to no diabetes. The primary outcome was occurrence of VTE. The model was adjusted for potential confounders for VTE.
Results
There were 7086 people with type 1 diabetes and 95,566 with type 2 diabetes, diagnosed before 1 January 2009. The non‐diabetes group consisted of 1,407,699 people. In the unadjusted analysis, there was no increased risk of VTE with type 1 diabetes (HR 1.00, 95% CI 0.76–1.33) but there was for type 2 diabetes (HR 2.70, 95% CI 2.57–2.84). In the fully adjusted model, VTE risk was increased in type 1 diabetes (HR 1.46, 95% CI 1.11–1.92), but not with type 2 diabetes (HR 1.06, 95% CI 0.98–1.14).
Conclusions
Type 1 diabetes was associated with a greater risk for VTE while type 2 diabetes was not. Further work is needed to determine the reason(s) for this.
Keywords: cohort study, computerised medical records, primary care, type 1 diabetes, type 2 diabetes, venous thromboembolism
What’s new
What is already known
Diabetes increases the risk for arterial atherosclerotic disease. Whether diabetes increases the risk for venous thromboembolism (VTE) is uncertain and may relate to failure to distinguish type 1 from type 2 diabetes.
What this study has found
Diabetes increases the risk for arterial atherosclerotic disease. Whether diabetes increases the risk for venous thromboembolism (VTE) is uncertain and may relate to failure to distinguish type 1 from type 2 diabetes.
What are the clinical implications of the study
Type 1 diabetes should be considered an at‐risk group for VTE. The mechanism(s) underlying this requires further investigation. Attention should be paid to the type of diabetes when performing and analysing clinical studies on antithrombotic drugs.
1. INTRODUCTION
Diabetes carries an increased risk of complications from arterial atherothrombosis. 1 Important mediators for this are hypertension, smoking and dyslipidaemia. Diabetes is also considered a proinflammatory condition that itself may directly predispose to atherothrombosis. 1 The inflammatory cascade is also integral to venous thromboembolism (VTE) but whether diabetes carries a greater risk of VTE is less clear.
Animal studies and pre‐clinical work in humans have highlighted several pathways, centred on coagulation and platelet reactivity, by which diabetes would plausibly predispose to VTE. 2 Diabetes is associated with increased tissue factor (TF) expression which activates thrombin, 3 converting fibrinogen into fibrin. Increased cellular synthesis of plasminogen activator inhibitor‐1 (PAI‐1) and fibrinogen, and reduced production of tissue plasminogen activator (t‐PA), will augment the developing fibrin structure. Up‐regulation of glycoproteins Ib and IIb/IIIa in people with diabetes triggers thrombus formation via interaction with von Willebrand Factor (vWF) and fibrin molecules. 1 Endothelial dysfunction will precipitate the rupture of the endothelium leading to the release of microparticles, which accelerate thrombus development through expression of procoagulant phospholipids. 4 Changes in plasma protein concentrations as well as plasma glucose levels lead to an increase in plasma viscosity in type 1 diabetes 5 and in type 2 diabetes. 6 The result is that thrombin generation (followed by measuring levels of the thrombin–antithrombin complex) is increased in individuals with type 1 diabetes or type 2 diabetes. 7 , 8
A meta‐analysis in 2008 reported greater risk of VTE in people with diabetes 9 but later analyses, found little to no increased risk. 10 , 11 A limitation to these studies is that many did not differentiate type 1 diabetes and type 2 diabetes. This is important as there are key differences between type 1 diabetes and type 2 diabetes in the prothrombotic milieu. 1 , 12 , 13 PAI‐1 levels tend to be reduced in type 1 diabetes but raised (prothrombotic) in type 2 diabetes. HDL cholesterol (considered to have anticoagulant properties 14 ) is often unchanged in type 1 diabetes but reduced in type 2 diabetes. Conversely, plasma levels of non‐esterified fatty acids (which may cause endothelial dysfunction and destabilisation of the fibrin clot 15 ) are raised in proportion to fat mass 16 – more in keeping with type 2 diabetes. These considerations would support an adverse role for insulin resistance in VTE pathogenesis. 12 , 13 However, a recent retrospective study including almost 5000 people in Taiwan has suggested that type 1 diabetes is an independent risk factor for VTE – although this did not report on VTE rate in type 2 diabetes. 17
Therefore, we investigated whether diabetes is an independent risk factor for VTE. We hypothesised that the risk for VTE would be greater in type 2 diabetes than in type 1 diabetes.
2. METHODS
2.1. Study design
The study was a retrospective cohort study, of adults with and without diabetes, included in the Royal College of General Practitioners (RCGP) Research and Surveillance Centre (RSC) database.
2.2. Data source
This comprises data from a sentinel network of primary care practices (general practices) distributed across England, which is representative of the general population. 18
Until recently, clinical codes were recorded using the Read coding system (Read Version 2 and Clinical Terms Version 3 [CTV3]), which includes codes for diagnoses, medications, investigations and processes of care. In 2018, the Read classification was replaced by the Systematized Nomenclature of Medicine Clinical Terms (SNOMED CT). 19
2.3. Study population
We used a two‐step process to identify type 1 diabetes and type 2 diabetes. 20 Step one identifies all people with diabetes (of any type), defined as those who had a diagnostic code (diagnosis of diabetes), clinical investigations (two or more fasted glucose, random glucose or glucose tolerance test values or HbA1c measurements consistent with diagnosis) or medication use (two or more prescriptions for oral diabetes medications [excluding metformin] or injectable therapies). Step two is the categorisation by diabetes type using a clinically based seven‐step algorithm. This accounts for codes for type of diabetes, age (at first insulin prescription), body mass index (BMI) and other key factors to determine type 1 diabetes or type 2 diabetes. 20 This step‐wise categorisation algorithm allows over‐riding of diagnostic codes where clinical characteristics are highly likely to indicate a specific type of diabetes. BMI is the final step.
We included all adults (age ≥18 years) in the RCGP RSC database prior to (1 January 2009).
2.4. Exposure and outcome measures
We report the relationship of diabetes type to VTE outcomes. The following variables were identified from the literature as being potential confounders of the relationship of diabetes to VTE and were therefore adjusted for: age, sex, BMI, smoking, CKD (Stage 3–5), metformin, statin and aspirin therapy, presence of cancer, hormone replacement therapy, oral contraception and atrial fibrillation (AF) as a proxy for anticoagulation. 1 , 7 , 8 , 12 , 13 , 17 , 21 The most recently recorded entry (prior to 2009) was utilised. Comorbidities were defined using clinical codes for diagnosis, investigation and process of care. The primary outcome was VTE, including deep vein thrombosis (DVT) and pulmonary embolus (PE), codes are listed in Appendix S1. Ethnicity was defined using the Office of National Statistics (ONS) official UK ethnicity categories: this defines five major ethnic categories: White, Mixed/multiple ethnic groups (e.g. White and Black Caribbean), Asian (including Indian, Pakistani, Bangladeshi and Chinese), Black (including African and Caribbean) and Other (including Arab and other minority groups not classified elsewhere). 18
2.5. Statistical Methods
Continuous and categorical variables were presented as means, or proportions, as appropriate. Persons were subject to follow‐up from the index date (1 January 2009) until the first diagnosis of VTE, withdrawal from the RCGP RSC, or 31 December 2018. At start of follow‐up (1 January 2009) we assessed whether people had type 1 diabetes or type 2 diabetes, we did not perform a time‐varying covariate analyses (e.g. in case people developed diabetes during follow‐up, they did not switch from the no diabetes to the diabetes group). We calculated the incidence rate of VTE (per 10,000 person‐years) as the number of VTE cases divided by the person‐time at risk for both the diabetes and non‐diabetes groups.
Hazard ratios (HR) and 95% confidence intervals (CI) were calculated for the association between diabetes and VTE using Cox regression analyses. An unadjusted and adjusted analysis was performed to correct for potential confounders (as stated above). Using life table techniques, Kaplan‐Meier curves were generated to illustrate survival free from VTE in people with type 1 diabetes, type 2 diabetes and without diabetes. All analyses were performed using R statistical software version 3.5.3.
2.6. Sensitivity analysis
Our primary analyses consisted of a complete case analysis. To verify these results, we repeated the Cox regression following multiple imputation for missing data. In addition, we excluded all people who developed diabetes during follow‐up (people free from diabetes on 1 January 2009 who developed diabetes thereafter), and also performed an analysis with the index date 1 January 2014; after which the Cox regression analyses were repeated.
2.7. Ethical considerations
All data were pseudonymised at the point of data extraction. No clinically identifiable information was available to researchers. The study was categorised as a service evaluation when assessed using the Health Research Authority (HRA) Medical Research Council (MRC) tool for identifying research. The study was also checked according to the University of Surrey Research Integrity and Governance Office Self‐Assessment for Governance and Ethics tool, which advised that a formal ethics review was not required. Approvals for the use of the data were acquired from the RCGP RSC approvals committee.
3. RESULTS
From the total RCGP RSC cohort of 1,510,351 adults from 293 primary care practices, 102,652 (7%) people were identified as having diabetes before 1 January 2009. Of these 95,566 (93%) were categorised as type 2 diabetes.
Mean follow‐up time was 7.6 years. In total there were 50 VTE events in the type 1 diabetes group (crude incidence 9.6 per 10,000 person years), 1,798 events in type 2 diabetes (crude rate 25.8 per 10,000 person years) and 10,166 events (9.5 per 10,000 person years) in non‐diabetes group. The characteristics of the diabetes and non‐diabetes populations are shown in Table 1.
Table 1.
Baseline characteristics of people with and without diabetes.
| No diabetes (n = 1,407,699) | Type 1 diabetes (n = 7086) | Type 2 diabetes (n = 95,566) | |
|---|---|---|---|
| Age (mean, SD) | 54.9 (18.9) | 49.8 (16.9) | 73.9 (13.3) |
| Male | 49% | 56% | 55% |
| HbA1c (mmol/mol) | 39 (7) | 70 (19) | 56 (16) |
| HbA1c (%) | 5.7 (0.7) | 8.5 (1.8) | 7.3 (1.5) |
| Body mass index (mean, SD) | 25.8 (5.5) | 26.4 (5.3) | 30.5 (6.4) |
| Smoking status | |||
| Non‐smoker | 785,147 56% | 3,908 55% | 44,988 47% |
| Ex‐smoker | 264,598 19% | 1,303 18% | 30,649 32% |
| Active‐smoker | 357,954 25% | 1,875 27% | 19,929 21% |
| Venous thromboembolism (any type) | 10,166 | 50 | 1,798 |
| Venous thromboembolism | |||
| Unspecified (n) | 196 | 2 | 34 |
| DVT (n) | 5386 | 32 | 965 |
| PE (n) | 4584 | 16 | 799 |
| Atrial fibrillation | 25,188 1.8% | 66 0.9% | 6,671 7.0% |
| Chronic kidney disease | 57,158 4.1% | 604 8.5% | 19,449 20% |
| Aspirin | 132,153 9.4% | 2,146 30% | 53,778 56% |
| Statin | 169,477 12% | 3,376 48% | 74,126 78% |
| Metformin | 4,734 0.3% | 775 11% | 60,111 63% |
| Ethnicity a | |||
| Asian | 63,314 | 191 | 7796 |
| Black | 31,847 | 118 | 2779 |
| Mixed | 9489 | 48 | 530 |
| Other | 9267 | 24 | 448 |
| White | 917,278 | 5228 | 66,852 |
Ethnicity missing for 395.142 individuals.
In the unadjusted analysis, diabetes increased risk of VTE (HR 2.58, 95% CI 2.46–2.71) (Table 2); adjusted for age and sex, the HR was 1.31 (95% CI 1.25–1.38). However, in the fully adjusted model (adjusted for age, sex, BMI, smoking status, CKD, metformin, statin or aspirin use and atrial fibrillation), there was no increased risk of VTE (HR 1.07, 95% CI 0.99–1.15). The HRs for VTE, with incremental adjustment for covariates, are shown in Figure 1. Kaplan‐Meier curves (unadjusted analysis) illustrating freedom from VTE in people with type 1 diabetes, type 2 diabetes and people without diabetes are shown in Figure 2.
Table 2.
Unadjusted and adjusted hazard ratios for diabetes type and VTE.
| Covariates | No diabetes | Diabetes HR (95% CI) | Type 1 diabetes HR (95% CI) | Type 2 diabetes HR (95% CI) |
|---|---|---|---|---|
| Unadjusted | Reference | 2.58 (2.46–2.71) | 1.00 (0.76–1.33) | 2.70 (2.57–2.84) |
| Age and sex | Reference | 1.31 (1.25–1.38) | 1.55 (1.18–2.05) | 1.31 (1.24–1.38) |
| Fully adjusted model a | Reference | 1.07 (0.99–1.15) | 1.46 (1.11–1.92) | 1.06 (0.98–1.14) |
Includes age, sex, body mass index, smoking status, chronic kidney disease, aspirin, metformin, statins and AF.
Figure 1.
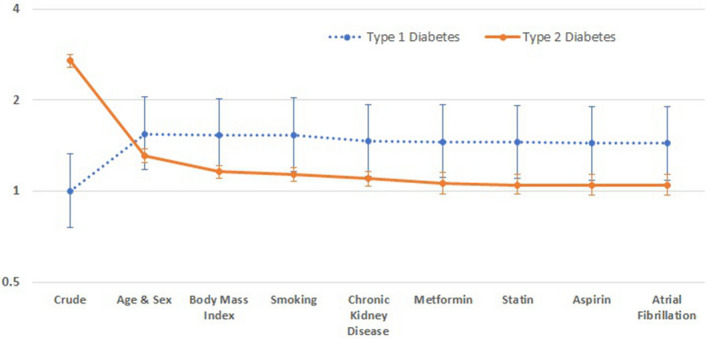
Hazard ratios for VTE, with incremental adjustment for covariates. AF, atrial fibrillation; BMI, body mass index; CKD, chronic kidney disease.
Figure 2.
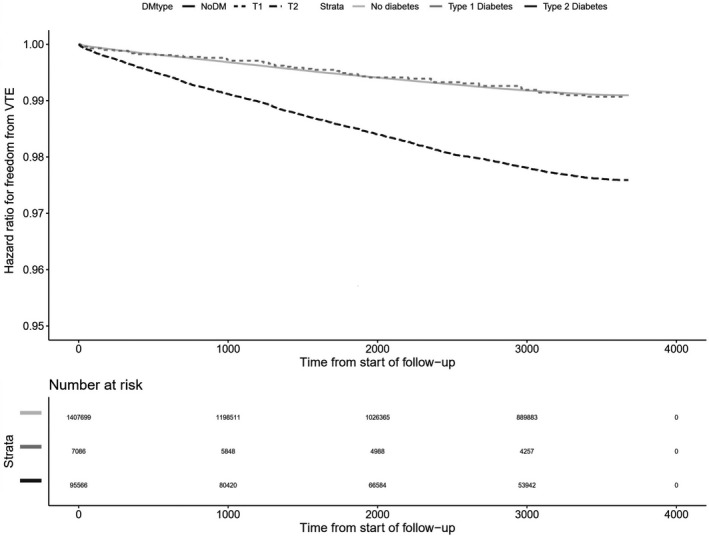
Unadjusted Kaplan Meier curves illustrating freedom from venous thromboembolism (VTE) in people with type 1 diabetes, type 2 diabetes and people without diabetes.
When considering diabetes type, type 2 diabetes was associated with VTE after adjustment for age and sex HR 1.31 (95% CI 1.24 – 1.38) but was not associated with increased risk of VTE in the fully adjusted model (HR 1.06, 95% 0.98 – 1.14). However, the risk of VTE was increased in people type 1 diabetes in the fully adjusted model (HR 1.46, 95% CI 1.11–1.92). Additional adjustment for the presence of cancer, oral contraception or HRT use did not change the relationships of diabetes with VTE – type 1 diabetes HR 1.45 (95% CI 1.10–1.92), type 2 diabetes HR 1.05 (95% CI 0.97–1.13). Moreover, additional adjustment for pre‐existing VTE did not alter the results.
In a sensitivity analyses, excluding all people who developed diabetes during follow‐up, similar relationships were found (for type 1 diabetes, HR 1.46, 95%CI 1.11–1.93 and for type 2 diabetes HR 1.05, 95% CI 0.97–1.14). Similarly, taking the index date from 1 January 2014, no difference in outcome was found: Type 1 diabetes (n = 9558) HR 1.26 (95% CI 0.86–1.86), multivariable HR 2.01 (95% CI 1.37–2.97); type 2 diabetes (n = 134,885) HR 2.56 (95% CI 2.39–2.75), multivariable 1.14 (95% CI 1.02–1.27). As a final sensitivity analysis, we used multiple imputation for missing values for explanatory variables, which did not change the relationships found— for type 1 diabetes, the HR was 1.47 (95% CI 1.22–1.92) for the multivariable model. For type 2 diabetes, the imputed results were HR was 1.08 (95% CI 1.00–1.17) for the multivariable model.
4. DISCUSSION
Our data suggest a greater risk for VTE in people with type 1 diabetes compared to type 2 diabetes or controls. This was not anticipated. Although a recent publication showed a higher risk for VTE in type 1 diabetes than controls, it did not report rates in the type 2 diabetes population. 17
Although challenged by recent analysis of the Diabetes Control and Complications Trial (DCCT) and Epidemiology of Diabetes Interventions and Complications (EDIC) trial, 22 hyperglycaemia has traditionally been considered to contribute relatively little to the cardiovascular risk burden in diabetes. 23 Conversely, insulin resistance, adipose tissue dysfunction, inflammation and aberrant adipokine release have been implicated. 24 Similarly, insulin resistance has been proposed to have a role in venous thromboembolic disease. 12 , 13 We had therefore anticipated that type 2 diabetes would have a greater risk than type 1 diabetes. It may be that in these studies, insulin resistance was simply a surrogate for obesity, rather than an independent risk factor. We included BMI (a surrogate for obesity), as a covariate in our model which may contribute to difference in outcome. Alternatively, the widespread use of metformin (63% in the type 2 diabetes population), could have ameliorated the haemostatic consequence of insulin resistance, 25 although current metformin use was included in our fully adjusted model.
Other analyses have found that diabetes carries no independent risk of VTE. 10 , 11 However, type 2 diabetes accounts for over 90% of all diabetes and will therefore exert a proportionate impact on VTE risk in all cases of diabetes. Our work has shown no increased risk of VTE in individuals with type 2 diabetes compared to the population without diabetes, but that type 1 diabetes, when analysed separately, does so.
The 1.46‐fold greater VTE risk in type 1 diabetes that we report was far lower than the 5.33‐fold risk reported from a group in Taiwan. 17 The incidence of VTE in our no diabetes group was 9.5 per 10,000 person‐years, which is in line with published data 26 but greater than the background (no diabetes) rate of 2.62 per 10,000 person years reported by Peng et al 17 This would have contributed to the effect of narrowing the HR for the type 1 diabetes group in our study.
The mechanism(s) that underpin the greater rate of VTE in type 1 diabetes merits further study. Virchow's triad represents three elements, each of which may predispose to thrombosis: a hypercoagulable state, vascular wall injury or circulatory stasis. A hypercoagulable state has been reported in type 1 diabetes, with a panoply of procoagulant mediators being raised: including vWF, Factor V, VIII, X and XI—all of which may relate to chronic inflammation. 27 Glycation of plasminogen in diabetes reduces the fibrinolytic activity of plasmin, which would predispose to thrombosis. 28 Few studies have looked at whether type 1 diabetes and type 2 diabetes have a differential influence on fibrin clot parameters or microparticles in thrombosis. 27 Vascular wall injury would arise from surgical procedures. However, hospital episode data may not reach primary care records, meaning that operations or periods of immobility may go unreported. In surgical patients, the length of hospital stay is up to 45% higher in those with diabetes, 29 putting them at greater thrombotic risk from immobility. Circulatory stasis might also ensue from atrial fibrillation (for which we adjusted) or heart failure, 30 although incidence rates of heart failure hospitalisation are no higher in type 1 diabetes than type 2 diabetes. 31
4.1. Limitations
The large size of the cohort and the robust nature of the diabetes case finding and algorithm for classification are major advantages of our study. Data quality of the RCGP RSC is high, complete case and multiple imputed analyses were similar. Hyperglycaemia may mediate a prothrombotic effect in diabetes. 5 , 6 As with Peng et al, 17 the model was not adjusted for glycated haemoglobin, due to missing data.
Although we adjusted for BMI, we were unable to identify phenotypes of type 2 diabetes. Furthermore, clinical coding for NALFD is poor in primary care. 32 Given that NAFLD is independently associated with VTE, 33 our approximation of risk in type 2 diabetes may have benefitted from distinguishing risks to subgroups of type 2 diabetes.
We did not perform a time‐varying covariate analyses; this implicates that if people did not have diabetes at start of follow‐up, they were classified accordingly for the entire duration of follow‐up. This results in a slight overestimated baseline risk for VTE in the no diabetes group and consequently, a lower hazard for VTE for people with type 1 diabetes. However, a sensitivity analysis, excluding all people who developed diabetes during follow‐up, showed comparable results to our primary model.
Theoretically, sodium‐glucose cotransporter‐2 inhibitors (SGLT2i) could increase blood viscosity, which may further increase risk of VTE. SGLT2i were introduced towards the end of our study period meaning too few data for interpretation. A recent meta‐analysis that found no association between SGLT2i and risk of VTE among people with type 2 diabetes. 34 As prescribing of SGLT2i increases, re‐analysis for risk should be undertaken.
To conclude, diabetes was associated with increased risk of VTE in the unadjusted analysis. However, after adjustment for confounders, this effect was negated. Any trend to greater VTE in the entire diabetes cohort may stem from the type 1 diabetes population.
CONFLICT OF INTEREST
The authors have no conflict of interest.
AUTHOR CONTRIBUTIONS
WH data analysis, manuscript writing; BN; data analysis; SdL data acquisition, data analysis; BF, MDF and NM data analysis; RA conception and design of the work; MBW and LR: conception and design of the work, data analysis and manuscript writing. All authors were involved in manuscript revision and approved the final version.
Supporting information
Appendix S1
Appendix S2
ACKNOWLEDGEMENTS
The authors wish to thank Filipa Ferreira (senior project manager) and Julian Sherlock (SQL developer) at University of Oxford.
REFERENCES
- 1. Paneni F, Beckman JA, Creager MA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur Heart J. 2013;34:2436‐2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patti G, Cavallari I, Andreotti F, et al. Prevention of atherothrombotic events in patients with diabetes mellitus: from antithrombotic therapies to new‐generation glucose‐lowering drugs. Nat Rev Cardiol. 2019;16:113‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Soma P, Swanepoel AC, Bester J, Pretorius E. Tissue factor levels in type 2 diabetes mellitus. Inflamm Res. 2017;66:365‐368. [DOI] [PubMed] [Google Scholar]
- 4. Nomura S, Shimizu M. Clinical significance of procoagulant microparticles. J Intensive Care. 2015;3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mishra N, Singh N. Blood viscosity, lipid profile, and lipid peroxidation in type‐1 diabetic patients with good and poor glycemic control. N Am J Med Sci. 2013;5:562‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jax TW, Peters AJ, Plehn G, Schoebel FC. Hemostatic risk factors in patients with coronary artery disease and type 2 diabetes – a two year follow‐up of 243 patients. Cardiovasc Diabetol. 2009;8:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davì G, Gennaro F, Spatola A, et al. Thrombin‐antithrombin III complexes in type II diabetes mellitus. J Diabetes Complications. 1992;6:7‐11. [DOI] [PubMed] [Google Scholar]
- 8. Reverter JL, Reverter JC, Tàssies D, et al. Thrombomodulin and induced tissue factor expression on monocytes as markers of diabetic microangiopathy: a prospective study on hemostasis and lipoproteins in insulin‐dependent diabetes mellitus. Am J Hematol. 1997;56:93‐99. [DOI] [PubMed] [Google Scholar]
- 9. Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta‐analysis. Circulation. 2008;117:93‐102. [DOI] [PubMed] [Google Scholar]
- 10. Bell EJ, Folsom AR, Lutsey PL, et al. Diabetes mellitus and venous thromboembolism: A systematic review and meta‐analysis. Diabetes Res Clin Pract. 2016;111:10‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heit JA, Leibson CL, Ashrani AA, Petterson TM, Bailey KR, Melton LJ 3rd. Is diabetes mellitus an independent risk factor for venous thromboembolism? A population‐based case‐control study. Arterioscler Thromb Vasc Biol. 2009;29:1399‐1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grant PJ. Diabetes mellitus as a prothrombotic condition. J Intern Med. 2007;262:157‐172. [DOI] [PubMed] [Google Scholar]
- 13. Van Schouwenburg IM, Mahmoodi BK, Veeger N, et al. Insulin resistance and risk of venous thromboembolism: results of a population‐based cohort study. J Thromb Haemost. 2012;10:1012‐1018. [DOI] [PubMed] [Google Scholar]
- 14. Fernandez JA, Deguchi H, Banka CL, Witztum JL, Griffin JH. Re‐evaluation of the anticoagulant properties of high‐density lipoprotein‐brief report. Arterioscler Thromb Vasc Biol. 2015;35:570‐572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tanka‐Salamon A, Komorowicz E, Szabo L, Tenekedjiev K, Kolev K. Free fatty acids modulate thrombin mediated fibrin generation resulting in less stable clots. PLoS One. 2016;11:e0167806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes. 2011;60:2441‐2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Peng Y‐H, Lin Y‐S, Chen C‐H, et al. Type 1 diabetes is associated with an increased risk of venous thromboembolism: A retrospective population‐based cohort study. PLoS One. 2020;15:e0226997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Whyte MB, Hinton W, McGovern A, et al. Disparities in glycaemic control, monitoring, and treatment of type 2 diabetes in England: a retrospective cohort analysis. PLoS Med. 2019;16:e1002942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Digital N .SNOMED CT in primary care – implementation update.
- 20. McGovern A, Hinton W, Correa A, Munro N, Whyte M, de Lusignan S. Real‐world evidence studies into treatment adherence, thresholds for intervention and disparities in treatment in people with type 2 diabetes in the UK. BMJ Open. 2016;6:e012801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107:I9‐16. [DOI] [PubMed] [Google Scholar]
- 22. Bebu I, Schade D, Braffett B, et al. Risk factors for first and subsequent CVD events in type 1 diabetes: the DCCT/EDIC study. Diabetes Care. 2020;43:867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Group AC , Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560‐2572. [DOI] [PubMed] [Google Scholar]
- 24. Taube A, Schlich R, Sell H, Eckardt K, Eckel J. Inflammation and metabolic dysfunction: links to cardiovascular diseases. Am J Physiol Heart Circ Physiol. 2012;302:H2148‐2165. [DOI] [PubMed] [Google Scholar]
- 25. Lu DY, Huang CC, Huang PH, et al. Metformin use in patients with type 2 diabetes mellitus is associated with reduced risk of deep vein thrombosis: a non‐randomized, pair‐matched cohort study. BMC Cardiovasc Disord. 2014;14:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Spannagl M, Heinemann LA, Dominh T, Assmann A, Schramm W, Schurmann R. Comparison of incidence/risk of venous thromboembolism (VTE) among selected clinical and hereditary risk markers: a community‐based cohort study. Thromb J. 2005;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sobczak AIS, Stewart AJ. Coagulatory defects in type‐1 and type‐2 diabetes. Int J Mol Sci. 2019;20:6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ajjan RA, Gamlen T, Standeven KF, et al. Diabetes is associated with posttranslational modifications in plasminogen resulting in reduced plasmin generation and enzyme‐specific activity. Blood. 2013;122:134‐142. [DOI] [PubMed] [Google Scholar]
- 29. Levy N, Dhatariya K. Pre‐operative optimisation of the surgical patient with diagnosed and undiagnosed diabetes: a practical review. Anaesthesia. 2019;74(Suppl 1):58‐66. [DOI] [PubMed] [Google Scholar]
- 30. Tang L, Wu YY, Lip GY, Yin P, Hu Y. Heart failure and risk of venous thromboembolism: a systematic review and meta‐analysis. Lancet Haematol. 2016;3:e30–44. [DOI] [PubMed] [Google Scholar]
- 31. McAllister DA, Read SH, Kerssens J, et al. Incidence of hospitalization for heart failure and case‐fatality among 3.25 million people with and without diabetes mellitus. Circulation. 2018;138:2774‐2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alexander M, Loomis AK, Fairburn‐Beech J, et al. Real‐world data reveal a diagnostic gap in non‐alcoholic fatty liver disease. BMC Med. 2018;16:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Di Minno MN, Tufano A, Rusolillo A, Di Minno G, Tarantino G. High prevalence of nonalcoholic fatty liver in patients with idiopathic venous thromboembolism. World J Gastroenterol. 2010;16:6119‐6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang A, Yang K, Wang T, Zhang N, Tang H, Feng X. Effects of sodium‐glucose cotransporter 2 inhibitors on risk of venous thromboembolism in patients with type 2 diabetes: a systematic review and meta‐analysis. Diabetes Metab Res Rev. 2020;36:e3174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2


