Figure 1.
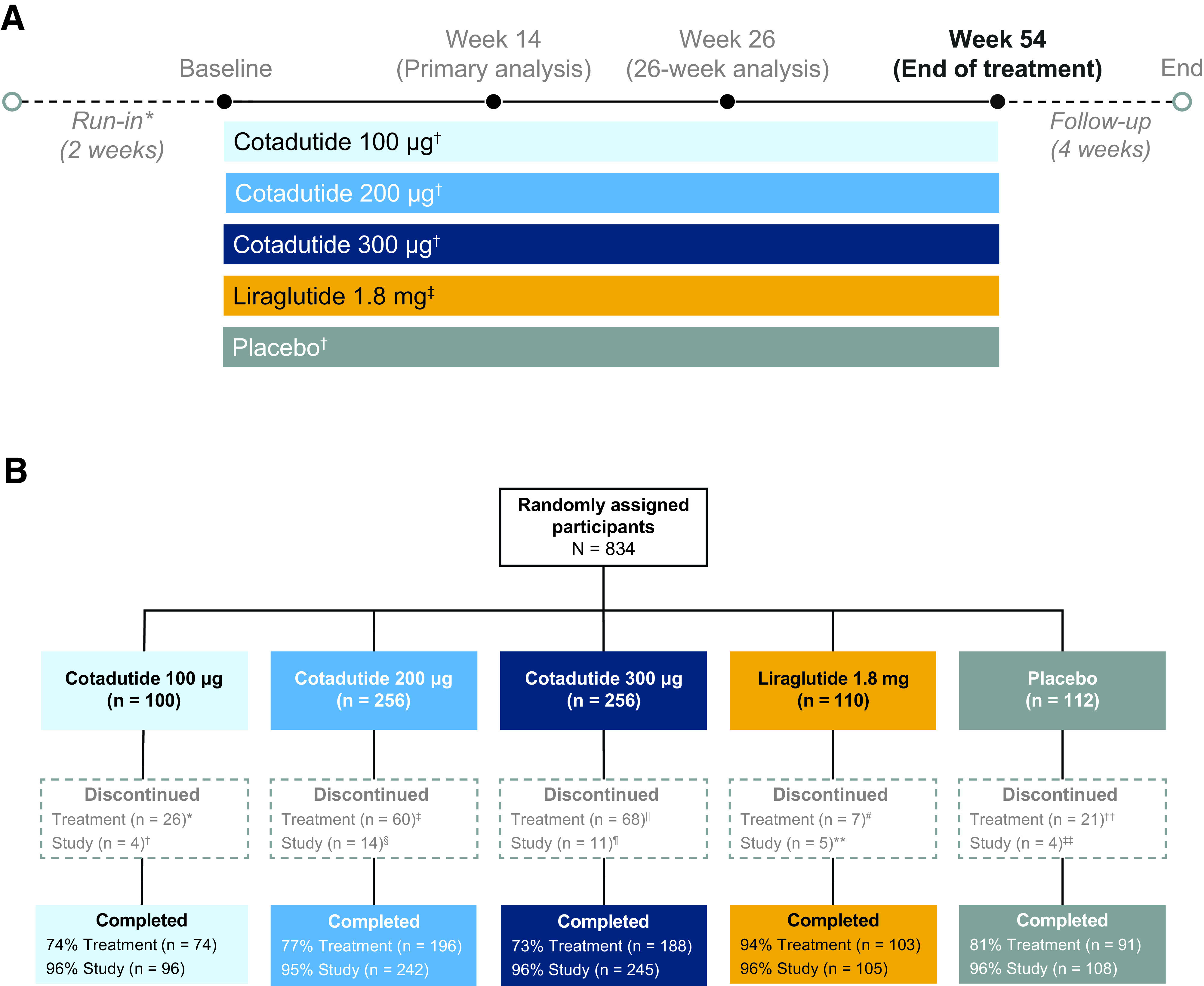
Study design and participant disposition. A: Cotadutide (double blind) and liraglutide (open-label) were given as once-daily subcutaneous injections. *Run-in was performed on a background of stable metformin treatment, which was maintained for the duration of the study. †Double-blinded, once-daily, subcutaneous injections. ‡Open-label, once-daily, subcutaneous injections. B: Participant disposition. *Due to adverse event (n = 13), lack of therapeutic response (n = 1), protocol violation (n = 1), subject decision (n = 7), other (n = 3), lost to follow-up (n = 1). †Due to adverse event (n = 1), lost to follow-up (n = 1), subject decision (n = 2). ‡Due to adverse event (n = 39), condition under investigation worsened (n = 1), lack of therapeutic response (n = 3), subject decision (n = 16), other (n = 1). §Due to adverse event (n = 4), death (n = 2), lost to follow-up (n = 2), subject decision (n = 5), other (n = 1). ǁDue to adverse event (n = 55), subject decision (n = 13). ¶Due to adverse event (n = 1), death (n = 1), subject decision (n = 7), other (n = 2). #Due to adverse event (n = 2), subject decision (n = 4), lost to follow-up (n = 1). **Due to subject decision (n = 2), lost to follow-up (n = 2) other (n = 1). ††Due to adverse event (n = 5), condition under investigation worsened (n = 1), lack of therapeutic response (n = 4), subject decision (n = 10), development of study-specific discontinuation criteria (n = 1). ‡‡Due to subject decision (n = 3), lost to follow-up (n = 1).
