Abstract
The molecular basis of the persistence of experienced T lymphocytes, also known as “memory T lymphocytes,” is still enigmatic. We are beginning to understand their considerable heterogeneity and topographic compartmentalization into memory T cells circulating through the body and those residing in a particular tissue. In some tissues, like murine spleen, subpopulations of memory T cells proliferating in the absence of antigen (homeostatic proliferation) have been described. Other populations are maintained resting in terms of transcription, mobility, and proliferation in dedicated survival niches organized by stromal cells. The survival of these memory T cells is conditional on being in such a niche, where they can persist for a lifetime. Circulating memory T lymphocytes of distinct immune responses slowly decline in numbers over time. The rules governing their entry into and exit from blood, as well as their lifestyle outside of the blood and their relation to resident memory T cells are poorly understood. Homeostasis of circulating, proliferating, and resting memory T cells is obviously controlled by different rheostats: tissue-exit and tissue-entry signals for circulating and proliferation-inducing signals for proliferating memory T cells. For tissue-resident, resting memory T cells, it is the availability of their survival niche. Apparently, this mechanism (i.e., the link between memory T cell and stromal cell) is so robust that it provides efficient T-cell memory over a lifetime in tissues such as the bone marrow.
COMPLEXITY AND COMPARTMENTALIZATION OF T-CELL MEMORY
More than 65 years ago, the pioneering work of McGregor and Gowans (1963) demonstrated that primary immune reactions are dependent on circulating lymphocytes, while secondary immune reactions are not. Yet, since then, the experimental dilemma of accessibility of circulating versus less accessible tissue-resident lymphocytes in humans and mice has confused our understanding of how immunological memory is organized and maintained. Moreover, antigenic experiences of mice and humans are clearly not directly comparable (Japp et al. 2017; Masopust et al. 2017). Blood-borne human memory lymphocytes and splenic murine memory lymphocytes have often been considered as representing the diversity and lifestyle of all memory lymphocytes. Information on how many lymphocytes are present in secondary lymphoid organs (SLOs), lymph, and nonlymphoid tissue is scarce. An estimation of the overall number of lymphocytes in nonlymphoid tissue of humans, including bone marrow, is about 1011, while twice as many have been estimated for secondary lymphoid tissues, blood, and lymph, but this estimation did not discriminate between different types of lymphocytes (Trepel 1974). It has been claimed that most of the T lymphocytes in the tissues are antigen-experienced, but they have not been exactly enumerated so far (Farber et al. 2014). About 0.8 to 8 × 108 antigen-experienced, CD45RO+ T lymphocytes per liter of human blood are circulating at any given time point (Bisset et al. 2004). Initial evidence that antigen-experienced T lymphocytes of distinct immune responses may have preferences to home to particular tissues, where they are detectable for long time periods, was generally interpreted as a topographic preference of circulating memory cells (Picker et al. 1990; Mackay et al. 1992, 1996; Tarazona et al. 1996; Austrup et al. 1997; Hogan et al. 2001; Marshall et al. 2001; Masopust et al. 2001; Reinhardt et al. 2001; Klonowski et al. 2004; Clark et al. 2006). However, ∼10 years ago, the concept of tissue-resident memory T lymphocytes was developed, claiming that at least some of the memory T lymphocytes present in a particular tissue are not circulating but permanent residents of that tissue (Gebhardt et al. 2009; Tokoyoda et al. 2009; Clark et al. 2012). Since then, the complex compartmentalization of immunological memory, its division of labor, and its redundancy is becoming more evident (Purwar et al. 2011; Teijaro et al. 2011; Clark et al. 2012; Jiang et al. 2012; Mackay et al. 2012, 2016; Hofmann et al. 2013; Sathaliyawala et al. 2013; Farber et al. 2014; Okhrimenko et al. 2014; Thome et al. 2014; Sercan-Alp et al. 2015; Kumar et al. 2017; Beura et al. 2018a, 2019; Bartolome-Casado et al. 2019; Siracusa et al. 2019). The conventional classification of memory T lymphocytes from the spleen and blood, based on their homing propensities (i.e., chemokine receptor expression into stem [Zhang et al. 2005; Gattinoni et al. 2011], central, and effector memory cells [Sallusto et al. 1999]), or on their functional imprinting (i.e., Th1, Th2, and Th17 cells [Löhning et al. 2002]) should be revised to take this additional compartmental heterogeneity into account (Fig. 1). It may be in any case soon obsolete, given our emerging ability to determine global transcriptomes and epigenomes of individual cells, and their antigen receptor repertoires. Clusters of related memory T cells and their trajectories will clearly define the heterogeneity of memory T cells on a global level (Durek et al. 2016; Youngblood et al. 2017). It will be a challenge to match such global molecular cluster classifications with the classical T-cell typology.
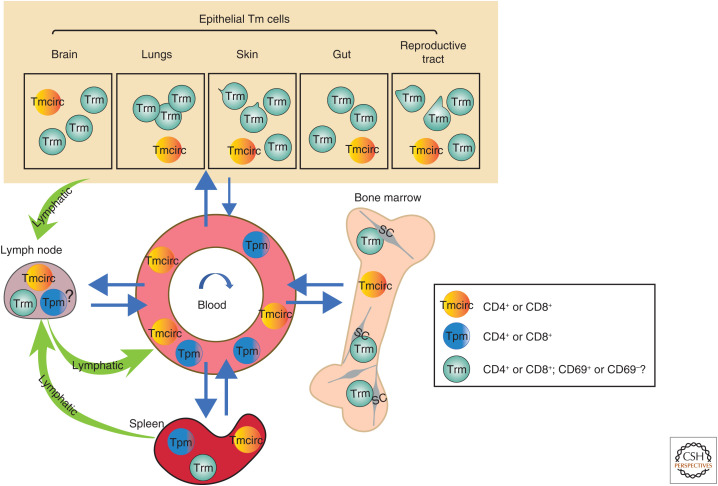
Figure 1.
Topography of circulating, proliferating, and resident memory T lymphocytes in steady state. The localization and migration of three memory T (Tm) lymphocyte cell subsets with different lifestyles (i.e., circulating [Tmcirc], proliferating [Tpm], and tissue-resident memory T [Trm] cells) and their presence in epithelial tissues, blood, secondary lymphoid organs, and bone marrow. In the skin and reproductive tract, motile Trm cells have been observed, while in bone marrow they are immobile. CD69 is a key marker that is expressed by Trm cells; however, Trm cells lacking expression of CD69 have also been suggested. (SC) Stromal cell.
T-CELL MEMORY IN TISSUE AND IN BLOOD
Determination of the persistence of T-cell memory is plagued by the basic problem of defining the memory phase of an immune response (i.e., separating T-cell-receptor-dependent from T-cell-receptor-independent maintenance of memory T cells). Defining the end of an immune reaction is difficult; it extends beyond the formation of germinal centers (MacLennan 1994). The duration of the late contraction phase of an immune response (i.e., when antigen is still present and experienced T and B lymphocytes are already circulating in the blood) is difficult to discriminate from the memory phase when no antigen is present. Upon withdrawal of antigen, CD8+ T cells have been shown to proliferate for up to eight more rounds, their survival promoted by cytokines like IL-2, -7, and -15 (Wong and Pamer 2001). Antigen-specific T cells are obviously not stimulated by antigen-presenting cells while circulating in the blood. Memory CD8+ T cells generated by vaccination against yellow fever virus (YFV) have been shown to circulate in the blood of human vaccinees for at least 2 yr after vaccination, most but not all of them resting in terms of proliferation (Akondy et al. 2017). In mice, “half-lives” of circulating antigen-experienced T lymphocytes (i.e., their numerical decrement by 50% in the blood or spleen) of <21 d have been reported for intentional immunizations (Tokoyoda et al. 2009), and 15–70 d for adoptively transferred experienced splenic T lymphocytes (Harrington et al. 2008; Lohning et al. 2008). It should be noted that in the original work of McGregor and Gowans, thoracic duct drainage of rats immunized against tetanus toxoid 3 wk before had no effect on the secondary immune response to the toxin, suggesting that most memory lymphocytes had been circulating for <3 wk, if at all. For murine memory CD4+ T lymphocytes, quantitative persistence in the spleen for >100 d has been demonstrated, upon conditional inactivation of the gene encoding the T-cell antigen receptor cα chain (Polic et al. 2001). Likewise, CD4+ murine T lymphocytes generated in vitro and adoptively transferred into MHC class II–deficient naive hosts (i.e., unable to present antigen to CD4+ T cells) persisted over 70 d in the spleen (Swain et al. 1999). Memory CD8+ T lymphocytes, upon genetic ablation of the TCR cα chain encoding genes, slowly declined in numbers over a time frame of 100 d. This might reflect the dependency of a CD122− subpopulation of splenic memory CD8+ T cells on T-cell receptor stimulation, as it has been shown that adoptively transferred CD122− lymph node T cells require expression of MHC class I by their hosts, while CD122+ memory T cells do not (Boyman et al. 2006).
For antigen-experienced human T lymphocytes, persistence in the absence of the original or a cross-reactive antigen is difficult to prove. Memory T cells generated in humans by vaccination against smallpox have been shown to disappear from the blood with half-lives of 8–15 yr (Hammarlund et al. 2003). Interestingly, it has been shown that CD4+ memory T cells reactive to childhood pathogens like measles, mumps, and rubella are rare if not absent in the blood of aged individuals, who presumably had encountered these antigens decades ago as children. These persist in the same individuals in the bone marrow (Okhrimenko et al. 2014) at frequencies and absolute numbers equal to CD4+ memory T cells specific for recurrent antigens like tetanus or persistent antigens like cytomegalovirus memory T cells, which also persist in the blood. The absence of measles-, mumps-, or rubella-specific memory T cells from the blood per se clearly argues that the memory cells specific for these pathogens in the bone marrow are truly resident cells. Interestingly, those memory T cells of the bone marrow are “polyfunctional” (Betts et al. 2006; Seder et al. 2008; Okhrimenko et al. 2014) (i.e., able to express multiple effector cytokines efficiently when restimulated by antigen-presenting cells of their host). Thus, the disappearance of memory T cells from the blood over time after their generation does not necessarily reflect either their disappearance from the host or “immune aging” (i.e., loss of functional memory) (Fig. 2).
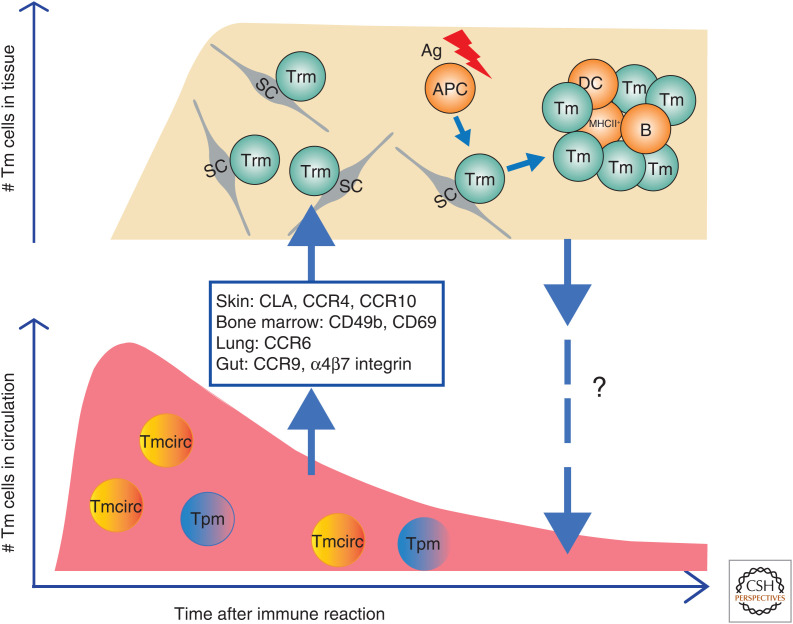
Figure 2.
Dynamics of memory T-cell populations over time. After an immune reaction, precursors of Trm migrate from blood and SLOs to their target tissue guided by chemokines and adhesion molecules as indicated and differentiate into Trm cells. In circulation, numbers of Tmcirc and Tpm cells gradually decline over time, while numbers of Trm cells initially increase and then presumably are maintained at stable levels. Upon antigenic challenge, Trm cells are reactivated by antigen-presenting cells (APCs), scanning them, and form nonconventional immune clusters in their host tissue and proliferate. To what extent reactivated Trm cells enter the circulation and participate in systemic immune responses is not quite clear. (SC) Stromal cell, (DC) dendritic cell.
The vast discrepancy in the persistence of memory T lymphocytes in the circulation and lymph between rodents and humans is obvious and not clearly understood so far. Adding to this confusion is the fact that there is only limited data detailing the entry and exit rates of lymphocytes into circulation, which have mainly been obtained via the administration of the compound FTY720 (fingolimod). FTY720 is an analog of sphingosine-1-phosphate (S1P) and therefore competes with it for the binding to sphingosine-1-phosphate receptor (S1PR1) (Brinkmann et al. 2002; Mandala et al. 2002; Matloubian et al. 2004), inhibiting the egress of lymphocytes from SLOs into the blood (Matloubian et al. 2004). In humans, treatment with the drug FTY720 resulted in a significant reduction in the number of central memory T cells within 2 wk; incidentally, this was the earliest time point analyzed. The number of effector memory T cells was less affected (Song et al. 2015), presumably because they express less S1PR1, and instead rely more on S1PR2 and 4 (Drouillard et al. 2018). Conversely, when fingolimod was withdrawn, the number of central memory T cells recovered within 2 wk (Claes et al. 2014; Ghadiri et al. 2017). For rodents, Gesner and Gowans (1962) showed that after 4 d of thoracic duct drainage of rats, the pool of circulating lymphocytes had been depleted. Likewise, the inhibition of S1P-mediated entry of T lymphocytes into the blood by the drug FTY720 in mice eliminated >90% of the circulating CD8+ and CD4+ lymphocytes within 3–4 d. This suggests that (1) circulating T lymphocytes are a distinct population of finite size, and (2) that they circulate in the blood for only a short time, until returning to tissue where they need another exit signal to eventually enter the circulation once more (Siracusa et al. 2017, 2018). Tissue-resident memory T lymphocytes have been shown to down-regulate S1P receptors 1, 4, and 5 and the transcription factor KLF2, which induces S1PR1, and this mechanism has been postulated to be essential for their absence from circulation (Skon et al. 2013; Farber et al. 2014; Okhrimenko et al. 2014; Sercan-Alp et al. 2015; Mackay et al. 2016; Kumar et al. 2017; Siracusa et al. 2019).
Which tissues qualify as hubs for circulating memory lymphocytes is a matter of debate. The bone marrow, lymph nodes, and spleen have been nominated and discussed, and, indeed, the same tissues may host resident and circulating memory T lymphocytes (Alp and Radbruch 2016; Di Rosa and Gebhardt 2016; Sercan-Alp and Radbruch 2016; Pascutti et al. 2019; Siracusa et al. 2019). When adoptively transferred into antigen-free hosts, murine splenic memory CD8+ T lymphocytes efficiently home to various tissues, including the bone marrow, spleen, liver, lung, lymph nodes, and peripheral blood, with no apparent preference (Becker et al. 2005). On the contrary, memory CD4+ and CD8+ T lymphocytes from the skin, gut, and bone marrow, preferentially home back to these same organs following adoptive transfer, and this preference is dependent on homing receptors such as CLA (Berg et al. 1991; Clark et al. 2006), α4β7 and CCR9 (Mora et al. 2003), and CD49b and CD69 (Shinoda et al. 2012; Hanazawa et al. 2013).
Of particular interest in this context is CD69, a surface molecule expressed by activated T lymphocytes (Testi et al. 1989), and by significant proportions of memory T lymphocytes in tissues like skin (Clark et al. 2006), gut (Zhang and Bevan 2013; Stelma et al. 2017), lung (Purwar et al. 2011; Teijaro et al. 2011; Steinert et al. 2015), the female reproductive tract (Beura et al. 2018a), and bone marrow (Tokoyoda et al. 2009; Shinoda et al. 2012; Tokoyoda and Radbruch 2012; Hanazawa et al. 2013; Okhrimenko et al. 2014; Hayashizaki et al. 2016). CD69 has been reported to antagonize the expression of the S1P1 receptor on the surface of T lymphocytes by internalization, thus blocking egress of activated T lymphocytes into the blood (Shiow et al. 2006). CD69-expressing memory T lymphocytes of various tissues, including the bone marrow, down-regulate expression of S1PR1 as well as the transcription factor KLF2, which induces it, corroborating the notion that these cells are not circulating through the blood (Tokoyoda et al. 2009; Shinoda et al. 2012; Siracusa et al. 2019). Moreover, CD69 seems to be essential for memory T lymphocytes or their precursors to enter certain tissues, as has been shown for the bone marrow by genetic ablation and serological inhibition (Shinoda et al. 2012). This novel function of CD69 is apparently independent of its interaction with S1PR1, but rather may depend on CD69 as an adhesion receptor and its ligands myosin light chains 9 and 12, which are expressed around blood vessels of organs like the lungs and bone marrow (Hayashizaki et al. 2016). CD69+ memory CD4+ and CD8+ lymphocytes residing in tissues like lung and bone marrow have been shown to express characteristic gene expression signatures, defining them as bona fide tissue-resident memory cells both in man and mouse (Mackay et al. 2016; Kumar et al. 2017; Siracusa et al. 2019). These signatures include expression of the key transcription factor Hobit, while other essential transcription factors of memory T-cell development, like T-bet, Gata3, and Rorγt, are not preferentially expressed (Mackay et al. 2016). Interestingly, preliminary evidence suggests that while both CD69+ and CD69− memory CD4+ T lymphocytes from human bone marrow show an extent of global DNA demethylation reminiscent of circulating CCR7+ central memory T cells, the pattern of CD69+ cells is clearly distinct from that of CD69− or circulating memory T cells (Durek et al. 2016). Whether or not CD69− memory CD4+ and CD8+ T lymphocytes of those tissues are tissue-resident or circulating (Steinert et al. 2015; Walsh et al. 2019) remains to be shown (e.g., by comparing their antigen-receptor repertoire).
PERSISTENCE OF MEMORY T CELLS—DORMANCY AND HOMEOSTATIC PROLIFERATION
The durability of T-cell memory for a particular antigen is clearly dependent on the persistence of the memory T cells conferring this memory. In a strict sense, when experienced T lymphocytes are maintained in the presence of persisting antigen (e.g., in chronic viral infections, autoimmunity, and cancer), this is not necessarily “memory” (the maintenance of information in the absence of the original instruction). Nevertheless, the molecular mechanisms may be similar, in particular, if the experienced T cells do not perceive or process the antigenic information, because they are either anergic, topographically separated, or blocked by checkpoint inhibition. It has been a rewarding exercise to unravel the molecular adaptations of experienced T lymphocytes to the chronic presence of antigen, identifying points of selective interference to unleash or ablate such cells in murine models of chronic inflammation and infection (Wherry et al. 2007; Albrecht et al. 2008; Niesner et al. 2008; Maschmeyer et al. 2018).
Memory T lymphocytes can persist independently of antigen (Swain et al. 1999; Polic et al. 2001), either by homeostatic proliferation or as resting cells. The maintenance of antigen-experienced T lymphocytes by antigen-independent (Murali-Krishna et al. 1999; Swain et al. 1999) “homeostatic” proliferation was originally demonstrated for splenic CD8+CD44high T lymphocytes adoptively transferred into antigen-free hosts (Becker et al. 2005). Proliferating cells were detected according to incorporation of bromodeoxyuridine (BrdU) into their DNA, or by labeling them with carboxyfluorescein (CFSE) prior to transfer, with detection of proliferating cells assessed by their dilution of the label by flow cytometry. The transferred splenic cells homed to a variety of organs, including the lungs, liver, blood, and bone marrow, and within 25 d, 30%–60% of them had proliferated at least once in the organs analyzed (Becker et al. 2005). Furthermore, 3 d following adoptive transfer of CFSE-labeled splenic CD8 T cells, the group of Di Rosa observed that 30% of the transferred cells in the bone marrow had proliferated, as had 15% of the cells in the spleen (Parretta et al. 2008). These coherent results, independently obtained by several research groups, led to the concept that the number of memory T cells is maintained by a balance of cell death, with a half-life of memory T cells of <2 wk, and proliferation of memory T cells, with 50% of the cells dividing at least once within 2 wk. This proliferation was shown to be dependent on the cytokines IL-7 and IL-15 (Kondrack et al. 2003; Li et al. 2003; Seddon et al. 2003), addressing common-γ chain cytokine receptors and activating signal transducer of activated T cells (STATs), in particular STAT3 and STAT5 (Surh and Sprent 2008). Whereas IL-7 has been postulated to be essential for memory CD4+ cells (Kondrack et al. 2003; Li et al. 2003), adoptively transferred splenic memory CD8+ T cells rather depended on IL-15 (Becker et al. 2002; Goldrath et al. 2002; Judge et al. 2002).
Understanding the maintenance of T-cell memory by homeostatic proliferation provides several conceptual challenges. It assumes that T-cell memory is restless, in that memory T cells are proliferative and migratory, and constantly in quest of cells presenting them their antigen and/or vital cytokines. Furthermore, it remains unclear how the balance between life and death of an individual memory cell is determined at the molecular level (i.e., how migration, in particular entry into and exit from blood and lymph, is regulated in relation to the need to “fuel up”). How can such a fragile, mobility-based system maintain stable numbers of memory T cells over extended periods of time, decades in the case of human immune responses to measles or smallpox? The true nature of this circulating, restless T-cell memory remains to be unraveled.
The analysis of proliferation of memory T cells residing in organs other than the spleen has provided evidence conflicting with the concept of homeostatic proliferation. The initial description of bone marrow–resident CD4+ memory T cells showed that <8% of these cells incorporated BrdU within 2 d, and <0.4% were in the S/G2/M phases of cell cycle according to staining of their DNA with propidium iodide (PI) (Tokoyoda et al. 2009). This was later confirmed for memory CD8+ T cells (Sercan-Alp et al. 2015) and for human memory CD4+ and CD8+ T lymphocytes of bone marrow (Okhrimenko et al. 2014), spleen, and lungs (Kumar et al. 2017), using the proliferation marker Ki-67. This protein is expressed exclusively by proliferating cells and is not expressed by cells resting in the G0 phase of the cell cycle (Gerdes et al. 1984; Scholzen and Gerdes 2000). Less than 1% of CD69+ and 2% of CD69− memory T cells in the bone marrow expressed Ki-67 (Okhrimenko et al. 2014). Proliferative rest was confirmed by PI staining, showing that <0.5% of the cells were in the S, G2, or M phases of the cell cycle (Sercan-Alp et al. 2015). Remarkably, feeding mice with BrdU resulted in a marked increase in frequency of Ki-67+CD8+ bone marrow memory T cells to up to 70% within 2 wk, indicating that BrdU can activate resting, resident bone marrow memory CD8+ T cells to proliferate by an as yet unclear mechanism (Sercan-Alp et al. 2015), questioning previous claims on memory T-cell proliferation based on BrdU incorporation (Parretta et al. 2005, 2008).
Additional evidence for the dichotomy between restless, proliferating, and resting resident memory T lymphocytes has been provided by ablating proliferating T lymphocytes with the drug cyclophosphamide (Siracusa et al. 2017). Using a 14-d treatment regimen, 50% of the CD44high memory CD8+ T lymphocytes were ablated from the spleen, while numbers of memory CD8+ T lymphocytes from the bone marrow were not affected. This was the case for memory CD8+ T lymphocytes generated in an intentional immune response initiated 3 mo before onset of cyclophosphamide treatment and for the entire populations of memory CD8+ T lymphocytes of spleen and bone marrow (i.e., cells generated in unintentional immune reactions). Thus, the endogenous populations of bone marrow resident memory CD8+ T lymphocytes are not cyclophosphamide-sensitive (i.e., not proliferating). It should be noted that those cells efficiently had taken up cyclophosphamide; when reactivated ex vivo to proliferate, they died immediately.
Together with the earlier data from the groups of Ahmed and Di Rosa (Di Rosa and Santoni 2002; Becker et al. 2005; Parretta et al. 2005, 2008), demonstrating that 50% of splenic CD8 memory T lymphocytes may proliferate according to incorporation of BrdU or loss of the CFSE label, these data suggest that the murine spleen apparently hosts two types of memory CD8+ T lymphocytes at about equal share—proliferating and nonproliferating ones—and that this dichotomy is established as early as 3 mo after an immune response. This raises the question: which memory cells are circulating and which ones maintain long-term memory?
Evidence (McCune et al. 2000; Hellerstein et al. 2003; Westera et al. 2013; Ahmed et al. 2016) that circulating CD8+ T-cell memory is maintained by nonproliferating cells has been provided by labeling proliferating memory CD8+ T cells with deuterium (D2O) in humans vaccinated with YFV in a nonendemic area (Akondy et al. 2017). In this case, rapid proliferation of blood-borne CD8+ T lymphocytes was observed for the first 28 d after immunization, but from then on, and for the next 2 yr, proliferation rates dropped to 0.17% ± 0.9% per day. Deuterium labeling for 56 d in the memory phase of the immune response to YFV, starting 4–9 mo after vaccination, revealed 8.2% ± 2.6% of cells proliferating in these 2 mo. When labeled during the first 14 d of the immune reaction, the majority of circulating YFV-specific memory CD8+ T lymphocytes retained the deuterium label even after 2 yr, suggesting that the majority of circulating memory is maintained by nonproliferating cells. Interestingly, when bulk populations of circulating memory-phenotype cells were analyzed, higher rates of proliferation were observed (Westera et al. 2013), suggesting heterogeneity among circulating memory CD8+ (and CD4+) T cells, with significant proportions of newly generated and still proliferating memory cells. In line with this, YFV-specific memory CD8+ T cells disappeared from the blood of vaccinated persons at rates considerably higher than the proliferation rates over the time period of 2 yr (0.57% ± 0.25% per day) (Akondy et al. 2017). It remains unclear whether these cells had died or had left the blood to take residency in tissue (e.g., in the bone marrow) and then persisted there. In summary, human memory CD8 T cells circulating in the blood—mostly CD69− cells—contain a significant proportion of memory cells resting in terms of proliferation and persisting for more than 2 yr in the blood. In the spleen of mice, however, 50% of the CD69−CD44hi memory T lymphocytes continue to proliferate even 3 mo after they had been generated, while 50% do not proliferate at all, like their cousins resting in the bone marrow. Circulating and proliferating subpopulations of memory CD8+ T cells are not the same, and understanding the rules governing their homeostasis remains a challenge.
CONDITIONAL SURVIVAL IN MEMORY NICHES VERSUS INTRINSIC “HALF-LIFE” OF MEMORY T CELLS
When considering memory T lymphocytes as a more or less uniform population constantly circulating through the body, the calculation of “half-lives” describes the time period when 50% of the cells would have disappeared from circulation. For instance, the “half-life” of smallpox-specific memory CD8+ T cells from human blood has been calculated to be 8–15 yr (Hammarlund et al. 2003). The half-life of adoptively transferred or endogenous memory T cells specific for lymphocytic choriomeningitis virus (LCMV) on the other hand is only 15–70 d for murine spleen (Hammarlund et al. 2003; Lohning et al. 2008; Tokoyoda et al. 2009). The reason for this discrepancy is not clear, nor is the molecular mechanism maintaining the cells. In view of the apparent heterogeneity of memory T cells with respect to proliferation and maintenance, even in the same organ (e.g., the murine spleen, as discussed above), “half-lives” would have to be calculated separately for all subpopulations differing in their propensity to be maintained. Given our ignorance of the true heterogeneity of memory T cells in this respect, this seems an impossible task today. The term “half-life” is also a bit ambiguous, as it does not distinguish among (1) disappearance by death, (2) changing identity by proliferation and/or differentiation of cells, and (3) the emigration out of the area of observation. A relevant example for escape by emigration is the transition of memory T cells from SLOs and blood into their tissue of residency (e.g., the bone marrow) (Tokoyoda et al. 2009; Chang et al. 2018), a scenario by now mostly accepted in our understanding of the organization of immunological memory.
The concept of tissue residency of T-cell memory has fundamentally challenged the concept of “half-lives” of memory cells. It is obviously hard for a lymphocyte to avoid contact to other cells in tissue, and it is intriguing to speculate that interactions of memory lymphocytes with neighboring cells of the tissue impact on the organization and maintenance of the memory lymphocytes (Fig. 3). In murine bone marrow, numbers of memory CD4+ and CD8+ T cells generated in intentional immune responses remain constant over extended observation periods of up to 4 mo, as resident cells resting in terms of proliferation (Tokoyoda et al. 2009; Sercan-Alp et al. 2015; Siracusa et al. 2017). Given such longevity, “half-lives” are not appropriate to calculate as they would by far exceed the life span of a mouse. In essence, T-cell memory is quantitatively maintained for a lifetime in mice. For human bone marrow, analogous data on the maintenance of specific memory T cells over time are not available to our knowledge. So far, it has been shown that memory CD4+ T cells specific for childhood diseases, like measles, mumps, and rubella, are readily detectable in the bone marrow of aged humans (40–70 yr), at significant frequencies, while they are no longer detectable in their blood (Okhrimenko et al. 2014). These cells are resident, because they are not circulating in the blood, and resting, as they do not express Ki-67. Furthermore, they are clearly maintained for a lifetime. What is it that maintains tissue-resident memory T lymphocytes over such extensive time periods?
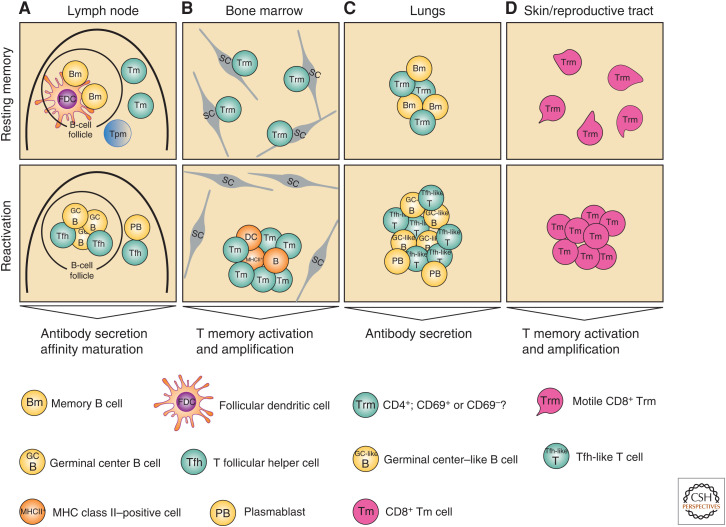
Figure 3.
Conventional and unconventional secondary immune reactions. (A) In secondary lymphoid organs like lymph nodes, memory T (Tm) cells are located in interfollicular regions and the subcapsular sinus. Upon reactivation, memory T cells regain a T follicular helper (Tfh) cell phenotype and control classical secondary immune reactions, resulting in the formation and affinity-maturation of antibody-secreting cells. (B) Resting Trm cells of the bone marrow are scattered over the parenchyma individually, each in close contact with a stromal cell (SC). They have to be scanned by antigen-presenting cells to become reactivated, then leave their niches and form immune clusters with the antigen-presenting cells, vigorously proliferating. (C) In the lungs, memory T cells persist in peribronchial clusters, in association with antigen-presenting cells, including B cells. These clusters might provide very fast reactivation of B and T cells in this barrier organ. (D) In the skin and reproductive tract, motile Trm cells have been described. In secondary immune responses, these motile Trm cells become immobile and proliferate.
This central question cannot be definitely answered today; however, it is likely that the environment of the memory lymphocytes delivers decisive survival signals. Survival of memory T cells would be conditional on being in the “right” environment, as we have discussed earlier for memory plasma cells (Manz and Radbruch 2002). In the bone marrow, individual memory CD4+ and CD8+ T lymphocytes are evenly spread out throughout the parenchyma, at frequencies of 0.1%–1% (Tokoyoda et al. 2009; Sercan-Alp et al. 2015; Siracusa et al. 2017). Most, if not all of them, are contacting mesenchymal stromal cells, a population of up to 5% of the bone marrow cells, which are quite diverse on the level of single-cell transcriptomes, with distinct subpopulations expressing either IL-7 or IL-15 (Tokoyoda et al. 2009; Sercan-Alp et al. 2015; Addo et al. 2019). Both memory CD4+ and CD8+ T lymphocytes are neighbors of IL-7-expressing stromal cells in the bone marrow (Tokoyoda et al. 2009; Sercan-Alp et al. 2015). Apparently, IL-7-expressing stromal cells of the bone marrow provide a “niche” for memory T lymphocytes, permitting them to individually persist over time. The restriction of one memory T cell per stromal cell implies that (1) the population size of the tissue-resident memory is determined by the number of available IL-7+ stromal cells, which in turn is determined by the volume of bone marrow and strictly related to the size of the individual, and (2) that once all niches are hosting a memory T cell, newly generated memory cells or their precursors will have to compete with preexisting ones for habitation of a niche. Moreover, because in the bone marrow of mice memory CD4+ and CD8+ T lymphocytes are apparently competing for the same niches, competition between memory CD4+ and CD8+ T cells is to be expected. For other tissues, additional cytokines have been discussed to provide survival signals for memory T cells. For example, for skin-resident memory T lymphocytes, activated TGF-β provided by keratinocytes has been invoked (Hirai et al. 2019). The cytokines IL-7, IL-15, type I interferons, and TGF-β have also been implied in the differentiation and/or maintenance of skin, gut, and lung tissue-resident memory T cells (Casey et al. 2012; Mackay et al. 2013; Adachi et al. 2015; Wakim et al. 2015).
The lifestyle of memory CD4+ T cells and especially their behavior in antigen recall responses may differ substantially among different organs (Fig. 3). In SLOs (Fig. 3A), memory CD4+ T cells reside in the interfollicular regions and subcapsular sinus where they constantly move to scan macrophages for their cognate antigen (Suan et al. 2015). Upon antigen reencounter, memory T cells rapidly regain a T follicular helper (Tfh) phenotype and drive the differentiation of memory B cells and the generation of plasmablasts (Weber et al. 2012; Moran et al. 2018). In contrast, memory CD4+ T cells in the bone marrow (Fig. 3B) are resting in terms of transcription, proliferation, and mobility (Siracusa et al. 2018). Upon cognate reactivation, they leave their niches and form “immune clusters,” together with MHC class II–expressing cells. However, they do not gain any follicular helper qualities. They proliferate vigorously for several days, then return to their niches and rest again (Siracusa et al. 2018). In the lungs (Fig. 3C), resident memory CD4+ T cells (Teijaro et al. 2011) also do not show evidence of activation (Kumar et al. 2017). They are in close contact with resident memory B cells (Allie et al. 2019) in spatially defined clusters (Vu Van et al. 2016), suggesting that they are also not migrating within the tissue. Both, antigen-specific T and B cells in the lung are rapidly reactivated in secondary immune responses and confer local protection (Teijaro et al. 2011; Onodera et al. 2012; Snyder and Farber 2019). Whether B-cell reactivation depends on help from lung-resident T cells is currently not known. Notably in the skin and in the female reproductive tract (Fig. 3D), steady-state resident memory CD8+ T cells were found to be motile and to patrol the tissue microenvironment (Beura et al. 2018a; Park et al. 2018). Following reinfection with cognate antigen, these Trm cells undergo rapid proliferation in situ to provide local protection (Beura et al. 2018a; Park et al. 2018). While circulating memory T cells in secondary lymphoid organs could scan for cells presenting them with cognate antigen, and then mount conventional secondary immune reactions, resident and resting memory T cells, at least memory CD4 T cells, since they do not contact MHC class II-expressing cells (Tokoyoda et al. 2009), have to be scanned by antigen-presenting cells in the tissue, resulting in unconventional secondary immune reactions, but probably also mobilizing them to participate in secondary immune reactions in secondary lymphoid organs (Behr et al. 2020). The relative contributions of circulating and tissue-resident memory T cells to efficient local and systemic protection must still be determined.
The situation may be complicated by heterogeneity of memory CD4+ and CD8+ T cells within the same tissue (Farber et al. 2014). Whereas memory CD4+ T cells in the lung are strictly tissue resident (Teijaro et al. 2011), some memory CD8+ T cells can migrate from lung to other tissues (Klonowski et al. 2004). Similarly, it has recently been shown for memory CD8+ T cells from the skin that some of them are not only highly motile within the tissue (Beura et al. 2018a) but that they can also migrate to the lymph nodes (Beura et al. 2018b).
At present, the molecular signals maintaining memory T lymphocytes in the bone marrow are not clear. For memory CD4+ T lymphocytes, it can be excluded that antigen plays a role, as they do not colocalize to MHC class II–expressing cells in the memory phase of the immune response (Tokoyoda et al. 2009). MHC class I–expressing cells are in the environment of memory CD8+ T cells, but they too are resting in terms of transcription and proliferation (Sercan-Alp et al. 2015), suggesting that antigen also does not play a role in their maintenance. The role of IL-7 and IL-15 remains enigmatic in the maintenance phase, while it is clear that IL-7 and IL-15 are essential for the establishment of T-cell memory in mice (Becker et al. 2002; Goldrath et al. 2002; Kondrack et al. 2003; Li et al. 2003; Seddon et al. 2003) and both memory CD4+ and CD8+ T cells colocalize with IL-7-expressing stromal cells (Addo et al. 2019). It has been suggested that other cells provide IL-15 to memory CD8+ T cells in the bone marrow (Herndler-Brandstetter et al. 2011). Whether it is IL-7 and IL-15, and/or other signals yet to be identified, stromal cells apparently organize resident T-cell memory and provide essential signals for conditional survival of the memory T cells as resting cells and thus the maintenance and persistence of T-cell memory over a lifetime.
CONCLUDING REMARKS
Memory T lymphocytes display an increasingly recognized diversity of lifestyles, and, consequently, homeostasis of different subpopulations is controlled by different rheostats (Fig. 4). For circulating memory T cells, it is obviously the tissue-entry and tissue-exit signals giving them access to survival signals; for proliferating memory T cells, it is the access to proliferation-inducing signals other than antigen. For tissue-resident and resting memory T cells, the rheostat apparently is the continued availability of the survival niche provided by distinct stromal cells. This mechanism is apparently a very robust one, maintaining functional memory T lymphocytes over a lifetime of the individual in tissues such as the bone marrow.
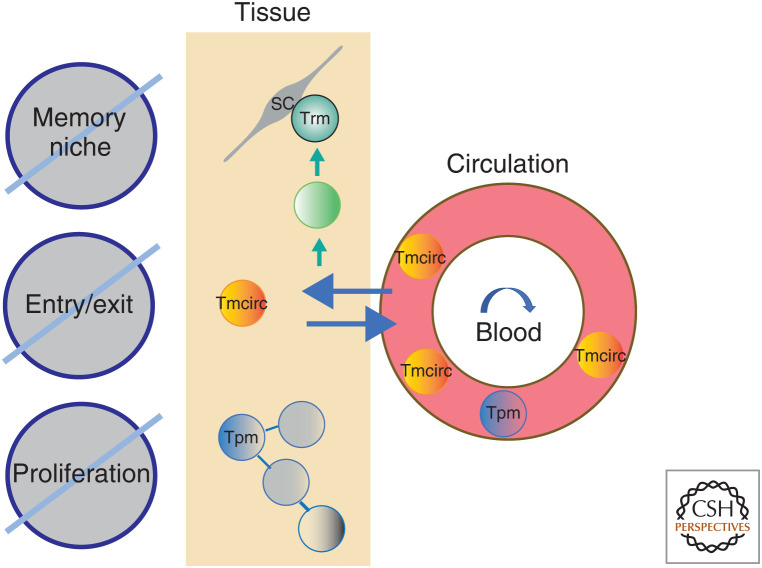
Figure 4.
Rheostats controlling the homeostasis of Tmcirc, Tpm, and Trm cells. According to their different lifestyles, different biological rheostats control the homeostasis of Tmcirc, Tpm, and Trm cells. Tissue entry and tissue exit regulates the homeostasis of Tmcirc cells, while for Tpm cells it is the availability of local proliferation-inducing signals. For Trm cells, it is obviously the continued availability of a survival niche as organized by a stromal cell (SC).
ACKNOWLEDGMENTS
This work was supported by the European Research Council (ERC) Advanced Grant 268987 (to A.R.), by the Deutsche Forschungsgemeinschaft ([DFG], German Research Foundation) and Project No. 389687267 (to J.D and A.R.), and by the Leibniz ScienceCampus Chronic Inflammation (www.chronische-entzuendung.org). F.S. was supported by Osteoimmune, an FP7 Marie Curie Initial Training Network (FP7-PEOPLE-2011-ITN-289150). H.-D.C. is supported by the Dr. Rolf M. Schwiete Foundation.
Footnotes
Editors: David Masopust and Rafi Ahmed
Additional Perspectives on T-Cell Memory available at www.cshperspectives.org
REFERENCES
- Adachi T, Kobayashi T, Sugihara E, Yamada T, Ikuta K, Pittaluga S, Saya H, Amagai M, Nagao K. 2015. Hair follicle-derived IL-7 and IL-15 mediate skin-resident memory T cell homeostasis and lymphoma. Nat Med 21: 1272–1279. 10.1038/nm.3962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addo RK, Heinrich F, Heinz GA, Schulz D, Sercan-Alp O, Lehmann K, Tran CL, Bardua M, Matz M, Lohning M, et al. 2019. Single-cell transcriptomes of murine bone marrow stromal cells reveal niche-associated heterogeneity. Eur J Immunol 48: 1372–1379. 10.1002/eji.201848053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R, Roger L, Costa Del Amo P, Miners KL, Jones RE, Boelen L, Fali T, Elemans M, Zhang Y, Appay V, et al. 2016. Human stem cell-like memory T cells are maintained in a state of dynamic flux. Cell Rep 17: 2811–2818. 10.1016/j.celrep.2016.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akondy RS, Fitch M, Edupuganti S, Yang S, Kissick HT, Li KW, Youngblood BA, Abdelsamed HA, McGuire DJ, Cohen KW, et al. 2017. Origin and differentiation of human memory CD8 T cells after vaccination. Nature 552: 362–367. 10.1038/nature24633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht I, Niesner U, Janke M, Radbruch A, Chang H. 2008. The pro-inflammatory immunological memory: twist1 as a marker for chronically activated T lymphocytes. Z Rheumatol 67: 684–688. 10.1007/s00393-008-0403-5 [DOI] [PubMed] [Google Scholar]
- Allie SR, Bradley JE, Mudunuru U, Schultz MD, Graf BA, Lund FE, Randall TD. 2019. The establishment of resident memory B cells in the lung requires local antigen encounter. Nat Immunol 20: 97–108. 10.1038/s41590-018-0260-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alp OS, Radbruch A. 2016. The lifestyle of memory CD8+ T cells. Nat Rev Immunol 16: 271. 10.1038/nri.2016.32 [DOI] [PubMed] [Google Scholar]
- Austrup F, Vestweber D, Borges E, Löhning M, Brauer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, et al. 1997. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflamed tissues. Nature 385: 81–83. 10.1038/385081a0 [DOI] [PubMed] [Google Scholar]
- Bartolome-Casado R, Landsverk OJB, Chauhan SK, Richter L, Phung D, Greiff V, Risnes LF, Yao Y, Neumann RS, Yaqub S, et al. 2019. Resident memory CD8 T cells persist for years in human small intestine. J Exp Med 216: 2412–2426. 10.1084/jem.20190414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, Ahmed R. 2002. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med 195: 1541–1548. 10.1084/jem.20020369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker TC, Coley SM, Wherry EJ, Ahmed R. 2005. Bone marrow is a preferred site for homeostatic proliferation of memory CD8 T cells. J Immunol 174: 1269–1273. 10.4049/jimmunol.174.3.1269 [DOI] [PubMed] [Google Scholar]
- Behr FM, Parga-Vidal L, Kragten NAM, van Dam TJP, Wesselink TH, Sheridan BS, Arens R, van Lier RAW, Stark R, van Gisbergen K. 2020. Tissue-resident memory CD8+ T cells shape local and systemic secondary T cell responses. Nat Immunol 21: 1070–1081. 10.1038/s41590-020-0723-4 [DOI] [PubMed] [Google Scholar]
- Berg EL, Yoshino T, Rott LS, Robinson MK, Warnock RA, Kishimoto TK, Picker LJ, Butcher EC. 1991. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med 174: 1461–1466. 10.1084/jem.174.6.1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, et al. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107: 4781–4789. 10.1182/blood-2005-12-4818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beura LK, Mitchell JS, Thompson EA, Schenkel JM, Mohammed J, Wijeyesinghe S, Fonseca R, Burbach BJ, Hickman HD, Vezys V, et al. 2018a. Intravital mucosal imaging of CD8+ resident memory T cells shows tissue-autonomous recall responses that amplify secondary memory. Nat Immunol 19: 173–182. 10.1038/s41590-017-0029-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beura LK, Wijeyesinghe S, Thompson EA, Macchietto MG, Rosato PC, Pierson MJ, Schenkel JM, Mitchell JS, Vezys V, Fife BT, et al. 2018b. T cells in nonlymphoid tissues give rise to lymph-node-resident memory T cells. Immunity 48: 327–338.e5. 10.1016/j.immuni.2018.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beura LK, Fares-Frederickson NJ, Steinert EM, Scott MC, Thompson EA, Fraser KA, Schenkel JM, Vezys V, Masopust D. 2019. CD4+ resident memory T cells dominate immunosurveillance and orchestrate local recall responses. J Exp Med 216: 1214–1229. 10.1084/jem.20181365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisset LR, Lung TL, Kaelin M, Ludwig E, Dubs RW. 2004. Reference values for peripheral blood lymphocyte phenotypes applicable to the healthy adult population in Switzerland. Eur J Haematol 72: 203–212. 10.1046/j.0902-4441.2003.00199.x [DOI] [PubMed] [Google Scholar]
- Boyman O, Cho JH, Tan JT, Surh CD, Sprent J. 2006. A major histocompatibility complex class I–dependent subset of memory phenotype CD8+ cells. J Exp Med 203: 1817–1825. 10.1084/jem.20052495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, et al. 2002. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem 277: 21453–21457. 10.1074/jbc.C200176200 [DOI] [PubMed] [Google Scholar]
- Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, Lucas PJ, Artis D, Wherry EJ, Hogquist K, et al. 2012. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol 188: 4866–4875. 10.4049/jimmunol.1200402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HD, Tokoyoda K, Radbruch A. 2018. Immunological memories of the bone marrow. Immunol Rev 283: 86–98. 10.1111/imr.12656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes N, Dhaeze T, Fraussen J, Broux B, Van Wijmeersch B, Stinissen P, Hupperts R, Hellings N, Somers V. 2014. Compositional changes of B and T cell subtypes during fingolimod treatment in multiple sclerosis patients: a 12-month follow-up study. PLoS ONE 9: e111115. 10.1371/journal.pone.0111115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K, Dowgiert RK, Kupper TS. 2006. The vast majority of CLA+ T cells are resident in normal skin. J Immunol 176: 4431–4439. 10.4049/jimmunol.176.7.4431 [DOI] [PubMed] [Google Scholar]
- Clark RA, Watanabe R, Teague JE, Schlapbach C, Tawa MC, Adams N, Dorosario AA, Chaney KS, Cutler CS, Leboeuf NR, et al. 2012. Skin effector memory T cells do not recirculate and provide immune protection in alemtuzumab-treated CTCL patients. Sci Transl Med 4: 117ra7. 10.1126/scitranslmed.3003008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Rosa F, Gebhardt T. 2016. Bone marrow T cells and the integrated functions of recirculating and tissue-resident memory T cells. Front Immunol 7: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Rosa F, Santoni A. 2002. Bone marrow CD8 T cells are in a different activation state than those in lymphoid periphery. Eur J Immunol 32: 1873–1880. [DOI] [PubMed] [Google Scholar]
- Drouillard A, Neyra A, Mathieu AL, Marçais A, Wencker M, Marvel J, Belot A, Walzer T. 2018. Human naive and memory T cells display opposite migratory responses to sphingosine-1 phosphate. J Immunol 200: 551–557. 10.4049/jimmunol.1701278 [DOI] [PubMed] [Google Scholar]
- Durek P, Nordström K, Gasparoni G, Salhab A, Kressler C, de Almeida M, Bassler K, Ulas T, Schmidt F, Xiong J, et al. 2016. Epigenomic profiling of human CD4+ T cells supports a linear differentiation model and highlights molecular regulators of memory development. Immunity 45: 1148–1161. 10.1016/j.immuni.2016.10.022 [DOI] [PubMed] [Google Scholar]
- Farber DL, Yudanin NA, Restifo NP. 2014. Human memory T cells: generation, compartmentalization and homeostasis. Nat Rev Immunol 14: 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, et al. 2011. A human memory T cell subset with stem cell–like properties. Nat Med 17: 1290–1297. 10.1038/nm.2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. 2009. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol 10: 524–530. 10.1038/ni.1718 [DOI] [PubMed] [Google Scholar]
- Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. 1984. Cell cycle analysis of a cell proliferation–associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 133: 1710–1715. [PubMed] [Google Scholar]
- Gesner BM, Gowans JL. 1962. The output of lymphocytes from the thoracic duct of unanaesthetized mice. Br J Exp Pathol 43: 424–430. [PMC free article] [PubMed] [Google Scholar]
- Ghadiri M, Fitz-Gerald L, Rezk A, Li R, Nyirenda M, Haegert D, Giacomini PS, Bar-Or A, Antel J. 2017. Reconstitution of the peripheral immune repertoire following withdrawal of fingolimod. Mult Scler 23: 1225–1232. 10.1177/1352458517713147 [DOI] [PubMed] [Google Scholar]
- Goldrath AW, Sivakumar PV, Glaccum M, Kennedy MK, Bevan MJ, Benoist C, Mathis D, Butz EA. 2002. Cytokine requirements for acute and Basal homeostatic proliferation of naive and memory CD8+ T cells. J Exp Med 195: 1515–1522. 10.1084/jem.20020033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. 2003. Duration of antiviral immunity after smallpox vaccination. Nat Med 9: 1131–1137. 10.1038/nm917 [DOI] [PubMed] [Google Scholar]
- Hanazawa A, Hayashizaki K, Shinoda K, Yagita H, Okumura K, Löhning M, Hara T, Tani-ichi S, Ikuta K, Eckes B, et al. 2013. CD49b-dependent establishment of T helper cell memory. Immunol Cell Biol 91: 524–531. 10.1038/icb.2013.36 [DOI] [PubMed] [Google Scholar]
- Harrington LE, Janowski KM, Oliver JR, Zajac AJ, Weaver CT. 2008. Memory CD4 T cells emerge from effector T-cell progenitors. Nature 452: 356–360. 10.1038/nature06672 [DOI] [PubMed] [Google Scholar]
- Hayashizaki K, Kimura MY, Tokoyoda K, Hosokawa H, Shinoda K, Hirahara K, Ichikawa T, Onodera A, Hanazawa A, Iwamura C, et al. 2016. Myosin light chains 9 and 12 are functional ligands for CD69 that regulate airway inflammation. Sci Immunol 1: eaaf9154. 10.1126/sciimmunol.aaf9154 [DOI] [PubMed] [Google Scholar]
- Hellerstein MK, Hoh RA, Hanley MB, Cesar D, Lee D, Neese RA, McCune JM. 2003. Subpopulations of long-lived and short-lived T cells in advanced HIV-1 infection. J Clin Invest 112: 956–966. 10.1172/JCI200317533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndler-Brandstetter D, Landgraf K, Jenewein B, Tzankov A, Brunauer R, Brunner S, Parson W, Kloss F, Gassner R, Lepperdinger G, et al. 2011. Human bone marrow hosts polyfunctional memory CD4+ and CD8+ T cells with close contact to IL-15–producing cells. J Immunol 186: 6965–6971. 10.4049/jimmunol.1100243 [DOI] [PubMed] [Google Scholar]
- Hirai T, Zenke Y, Yang Y, Bartholin L, Beura LK, Masopust D, Kaplan DH. 2019. Keratinocyte-mediated activation of the cytokine TGF-β maintains skin recirculating memory CD8+ T cells. Immunity 50: 1249–1261.e5. 10.1016/j.immuni.2019.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann M, Oschowitzer A, Kurzhals SR, Krüger CC, Pircher H. 2013. Thymus-resident memory CD8+ T cells mediate local immunity. Eur J Immunol 43: 2295–2304. 10.1002/eji.201343519 [DOI] [PubMed] [Google Scholar]
- Hogan RJ, Zhong W, Usherwood EJ, Cookenham T, Roberts AD, Woodland DL. 2001. Protection from respiratory virus infections can be mediated by antigen-specific CD4+ T cells that persist in the lungs. J Exp Med 193: 981–986. 10.1084/jem.193.8.981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japp AS, Hoffmann K, Schlickeiser S, Glauben R, Nikolaou C, Maecker HT, Braun J, Matzmohr N, Sawitzki B, Siegmund B, et al. 2017. Wild immunology assessed by multidimensional mass cytometry. Cytometry A 91: 85–95. 10.1002/cyto.a.22906 [DOI] [PubMed] [Google Scholar]
- Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. 2012. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature 483: 227–231. 10.1038/nature10851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge AD, Zhang X, Fujii H, Surh CD, Sprent J. 2002. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8+ T cells. J Exp Med 196: 935–946. 10.1084/jem.20020772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonowski KD, Williams KJ, Marzo AL, Blair DA, Lingenheld EG, Lefrançois L. 2004. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity 20: 551–562. 10.1016/S1074-7613(04)00103-7 [DOI] [PubMed] [Google Scholar]
- Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM. 2003. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med 198: 1797–1806. 10.1084/jem.20030735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar BV, Ma W, Miron M, Granot T, Guyer RS, Carpenter DJ, Senda T, Sun X, Ho SH, Lerner H, et al. 2017. Human tissue-resident memory T cells are defined by core transcriptional and functional signatures in lymphoid and mucosal sites. Cell Rep 20: 2921–2934. 10.1016/j.celrep.2017.08.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Huston G, Swain SL. 2003. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J Exp Med 198: 1807–1815. 10.1084/jem.20030725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löhning M, Richter A, Radbruch A. 2002. Cytokine memory of T helper lymphocytes. Adv Immunol 80: 115–181. 10.1016/S0065-2776(02)80014-1 [DOI] [PubMed] [Google Scholar]
- Löhning M, Hegazy AN, Pinschewer DD, Busse D, Lang KS, Höfer T, Radbruch A, Zinkernagel RM, Hengartner H. 2008. Long-lived virus-reactive memory T cells generated from purified cytokine-secreting T helper type 1 and type 2 effectors. J Exp Med 205: 53–61. 10.1084/jem.20071855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay CR, Marston WL, Dudler L, Spertini O, Tedder TF, Hein WR. 1992. Tissue-specific migration pathways by phenotypically distinct subpopulations of memory T cells. Eur J Immunol 22: 887–895. 10.1002/eji.1830220402 [DOI] [PubMed] [Google Scholar]
- Mackay CR, Andrew DP, Briskin M, Ringler DJ, Butcher EC. 1996. Phenotype, and migration properties of three major subsets of tissue homing T cells in sheep. Eur J Immunol 26: 2433–2439. 10.1002/eji.1830261025 [DOI] [PubMed] [Google Scholar]
- Mackay LK, Stock AT, Ma JZ, Jones CM, Kent SJ, Mueller SN, Heath WR, Carbone FR, Gebhardt T. 2012. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci 109: 7037–7042. 10.1073/pnas.1202288109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, Vega-Ramos J, Lauzurica P, Mueller N, Stefanovic T, et al. 2013. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Nat Immunol 14: 1294–1301. 10.1038/ni.2744 [DOI] [PubMed] [Google Scholar]
- Mackay LK, Minnich M, Kragten NA, Liao Y, Nota B, Seillet C, Zaid A, Man K, Preston S, Freestone D, et al. 2016. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 352: 459–463. 10.1126/science.aad2035 [DOI] [PubMed] [Google Scholar]
- MacLennan IC. 1994. Germinal centers. Annu Rev Immunol 12: 117–139. 10.1146/annurev.iy.12.040194.001001 [DOI] [PubMed] [Google Scholar]
- Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, et al. 2002. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 296: 346–349. 10.1126/science.1070238 [DOI] [PubMed] [Google Scholar]
- Manz RA, Radbruch A. 2002. Plasma cells for a lifetime? Eur J Immunol 32: 923–927. [DOI] [PubMed] [Google Scholar]
- Marshall DR, Turner SJ, Belz GT, Wingo S, Andreansky S, Sangster MY, Riberdy JM, Liu T, Tan M, Doherty PC. 2001. Measuring the diaspora for virus-specific CD8+ T cells. Proc Natl Acad Sci 98: 6313–6318. 10.1073/pnas.101132698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maschmeyer P, Petkau G, Siracusa F, Zimmermann J, Zugel F, Kuhl AA, Lehmann K, Schimmelpfennig S, Weber M, Haftmann C, et al. 2018. Selective targeting of pro-inflammatory Th1 cells by microRNA-148a-specific antagomirs in vivo. J Autoimmun 89: 41–52. 10.1016/j.jaut.2017.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Marzo AL, Lefrancois L. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291: 2413–2417. 10.1126/science.1058867 [DOI] [PubMed] [Google Scholar]
- Masopust D, Sivula CP, Jameson SC. 2017. Of mice, dirty mice, and men: using mice to understand human immunology. J Immunol 199: 383–388. 10.4049/jimmunol.1700453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. 2004. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427: 355–360. 10.1038/nature02284 [DOI] [PubMed] [Google Scholar]
- McCune JM, Hanley MB, Cesar D, Halvorsen R, Hoh R, Schmidt D, Wieder E, Deeks S, Siler S, Neese R, et al. 2000. Factors influencing T-cell turnover in HIV-1-seropositive patients. J Clin Invest 105: R1–8. 10.1172/JCI8647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor DD, Gowans JL. 1963. The antibody response of rats depleted of lymphocytes by chronic drainage from the thoracic duct. J Exp Med 117: 303–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, Von Andrian UH. 2003. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature 424: 88–93. 10.1038/nature01726 [DOI] [PubMed] [Google Scholar]
- Moran I, Nguyen A, Khoo WH, Butt D, Bourne K, Young C, Hermes JR, Biro M, Gracie G, Ma CS, et al. 2018. Memory B cells are reactivated in subcapsular proliferative foci of lymph nodes. Nat Commun 9: 3372. 10.1038/s41467-018-05772-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murali-Krishna K, Lau LL, Sambhara S, Lemonnier F, Altman J, Ahmed R. 1999. Persistence of memory CD8 T cells in MHC class I–deficient mice. Science 286: 1377–1381. 10.1126/science.286.5443.1377 [DOI] [PubMed] [Google Scholar]
- Niesner U, Albrecht I, Janke M, Doebis C, Loddenkemper C, Lexberg MH, Eulenburg K, Kreher S, Koeck J, Baumgrass R, et al. 2008. Autoregulation of Th1-mediated inflammation by twist1. J Exp Med 205: 1889–1901. 10.1084/jem.20072468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okhrimenko A, Grun JR, Westendorf K, Fang Z, Reinke S, von Roth P, Wassilew G, Kuhl AA, Kudernatsch R, Demski S, et al. 2014. Human memory T cells from the bone marrow are resting and maintain long-lasting systemic memory. Proc Natl Acad Sci 111: 9229–9234. 10.1073/pnas.1318731111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera T, Takahashi Y, Yokoi Y, Ato M, Kodama Y, Hachimura S, Kurosaki T, Kobayashi K. 2012. Memory B cells in the lung participate in protective humoral immune responses to pulmonary influenza virus reinfection. Proc Natl Acad Sci 109: 2485–2490. 10.1073/pnas.1115369109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SL, Zaid A, Hor JL, Christo SN, Prier JE, Davies B, Alexandre YO, Gregory JL, Russell TA, Gebhardt T, et al. 2018. Local proliferation maintains a stable pool of tissue-resident memory T cells after antiviral recall responses. Nat Immunol 19: 183–191. 10.1038/s41590-017-0027-5 [DOI] [PubMed] [Google Scholar]
- Parretta E, Cassese G, Barba P, Santoni A, Guardiola J, Di Rosa F. 2005. CD8 cell division maintaining cytotoxic memory occurs predominantly in the bone marrow. J Immunol 174: 7654–7664. 10.4049/jimmunol.174.12.7654 [DOI] [PubMed] [Google Scholar]
- Parretta E, Cassese G, Santoni A, Guardiola J, Vecchio A, Di Rosa F. 2008. Kinetics of in vivo proliferation and death of memory and naive CD8 T cells: parameter estimation based on 5-bromo-2′-deoxyuridine incorporation in spleen, lymph nodes, and bone marrow. J Immunol 180: 7230–7239. 10.4049/jimmunol.180.11.7230 [DOI] [PubMed] [Google Scholar]
- Pascutti MF, Geerman S, Collins N, Brasser G, Nota B, Stark R, Behr F, Oja A, Slot E, Panagioti E, et al. 2019. Peripheral and systemic antigens elicit an expandable pool of resident memory CD8+ T cells in the bone marrow. Eur J Immunol 49: 853–872. 10.1002/eji.201848003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker LJ, Terstappen LW, Rott LS, Streeter PR, Stein H, Butcher EC. 1990. Differential expression of homing-associated adhesion molecules by T cell subsets in man. J Immunol 145: 3247–3255. [PubMed] [Google Scholar]
- Polic B, Kunkel D, Scheffold A, Rajewsky K. 2001. How αβ T cells deal with induced TCR α ablation. Proc Natl Acad Sci 98: 8744–8749. 10.1073/pnas.141218898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purwar R, Campbell J, Murphy G, Richards WG, Clark RA, Kupper TS. 2011. Resident memory T cells (TRM) are abundant in human lung: diversity, function, and antigen specificity. PLoS ONE 6: e16245. 10.1371/journal.pone.0016245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. 2001. Visualizing the generation of memory CD4 T cells in the whole body. Nature 410: 101–105. 10.1038/35065111 [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401: 708–712. 10.1038/44385 [DOI] [PubMed] [Google Scholar]
- Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome JJ, Bickham KL, Lerner H, Goldstein M, Sykes M, et al. 2013. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity 38: 187–197. 10.1016/j.immuni.2012.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholzen T, Gerdes J. 2000. The Ki-67 protein: from the known and the unknown. J Cell Physiol 182: 311–322. [DOI] [PubMed] [Google Scholar]
- Seddon B, Tomlinson P, Zamoyska R. 2003. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol 4: 680–686. 10.1038/ni946 [DOI] [PubMed] [Google Scholar]
- Seder RA, Darrah PA, Roederer M. 2008. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol 8: 247–258. 10.1038/nri2274 [DOI] [PubMed] [Google Scholar]
- Sercan-Alp Ö, Radbruch A. 2016. Response: commentary: memory CD8+ T cells colocalize with IL-7+ stromal cells in bone marrow and rest in terms of proliferation and transcription. Front Immunol 7: 329. 10.3389/fimmu.2016.00329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sercan-Alp Ö, Durlanik S, Schulz D, McGrath M, Grün JR, Bardua M, Ikuta K, Sgouroudis E, Riedel R, Zehentmeier S, et al. 2015. Memory CD8+ T cells colocalize with IL-7+ stromal cells in bone marrow and rest in terms of proliferation and transcription. Eur J Immunol 45: 975–987. 10.1002/eji.201445295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda K, Tokoyoda K, Hanazawa A, Hayashizaki K, Zehentmeier S, Hosokawa H, Iwamura C, Koseki H, Tumes DJ, Radbruch A, et al. 2012. Type II membrane protein CD69 regulates the formation of resting T-helper memory. Proc Natl Acad Sci 109: 7409–7414. 10.1073/pnas.1118539109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiow LR, Rosen DB, Brdičková N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. 2006. CD69 acts downstream of interferon-α/β to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 440: 540–544. 10.1038/nature04606 [DOI] [PubMed] [Google Scholar]
- Siracusa F, Alp OS, Maschmeyer P, McGrath M, Mashreghi MF, Hojyo S, Chang HD, Tokoyoda K, Radbruch A. 2017. Maintenance of CD8+ memory T lymphocytes in the spleen but not in the bone marrow is dependent on proliferation. Eur J Immunol 47: 1900–1905. 10.1002/eji.201747063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siracusa F, McGrath MA, Maschmeyer P, Bardua M, Lehmann K, Heinz G, Durek P, Heinrich FF, Mashreghi MF, Chang HD, et al. 2018. Nonfollicular reactivation of bone marrow resident memory CD4 T cells in immune clusters of the bone marrow. Proc Natl Acad Sci 115: 1334–1339. 10.1073/pnas.1715618115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siracusa F, Durek P, McGrath MA, Sercan-Alp O, Rao A, Du W, Cendon C, Chang HD, Heinz GA, Mashreghi MF, et al. 2019. CD69+ memory T lymphocytes of the bone marrow and spleen express the signature transcripts of tissue-resident memory T lymphocytes. Eur J Immunol 49: 966–968. 10.1002/eji.201847982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skon CN, Lee JY, Anderson KG, Masopust D, Hogquist KA, Jameson SC. 2013. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol 14: 1285–1293. 10.1038/ni.2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder ME, Farber DL. 2019. Human lung tissue resident memory T cells in health and disease. Curr Opin Immunol 59: 101–108. 10.1016/j.coi.2019.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song ZY, Yamasaki R, Kawano Y, Sato S, Masaki K, Yoshimura S, Matsuse D, Murai H, Matsushita T, Kira J. 2015. Peripheral blood T cell dynamics predict relapse in multiple sclerosis patients on fingolimod. PLoS ONE 10: e0124923. 10.1371/journal.pone.0124923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert EM, Schenkel JM, Fraser KA, Beura LK, Manlove LS, Igyártó BZ, Southern PJ, Masopust D. 2015. Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell 161: 737–749. 10.1016/j.cell.2015.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelma F, de Niet A, Sinnige MJ, van Dort KA, van Gisbergen K, Verheij J, van Leeuwen EMM, Kootstra NA, Reesink HW. 2017. Human intrahepatic CD69+ CD8+ T cells have a tissue resident memory T cell phenotype with reduced cytolytic capacity. Sci Rep 7: 6172. 10.1038/s41598-017-06352-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suan D, Nguyen A, Moran I, Bourne K, Hermes JR, Arshi M, Hampton HR, Tomura M, Miwa Y, Kelleher AD, et al. 2015. T follicular helper cells have distinct modes of migration and molecular signatures in naive and memory immune responses. Immunity 42: 704–718. 10.1016/j.immuni.2015.03.002 [DOI] [PubMed] [Google Scholar]
- Surh CD, Sprent J. 2008. Homeostasis of naive and memory T cells. Immunity 29: 848–862. 10.1016/j.immuni.2008.11.002 [DOI] [PubMed] [Google Scholar]
- Swain SL, Hu H, Huston G. 1999. Class II–independent generation of CD4 memory T cells from effectors. Science 286: 1381–1383. 10.1126/science.286.5443.1381 [DOI] [PubMed] [Google Scholar]
- Tarazona R, Sponaas AM, Mavria G, Zhou M, Schulz R, Tomlinson P, Antoniou J, Mellor AL. 1996. Effects of different antigenic microenvironments on the course of CD8+ T cell responses in vivo. Int Immunol 8: 351–358. 10.1093/intimm/8.3.351 [DOI] [PubMed] [Google Scholar]
- Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrançois L, Farber DL. 2011. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol 187: 5510–5514. 10.4049/jimmunol.1102243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testi R, Phillips JH, Lanier LL. 1989. T cell activation via Leu-23 (CD69). J Immunol 143: 1123–1128. [PubMed] [Google Scholar]
- Thome JJC, Yudanin N, Ohmura Y, Kubota M, Grinshpun B, Sathaliyawala T, Kato T, Lerner H, Shen Y, Farber DL. 2014. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell 159: 814–828. 10.1016/j.cell.2014.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokoyoda K, Radbruch A. 2012. Signals controlling rest and reactivation of T helper memory lymphocytes in bone marrow. Cell Mol Life Sci 69: 1609–1613. 10.1007/s00018-012-0969-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokoyoda K, Zehentmeier S, Hegazy AN, Albrecht I, Grün JR, Löhning M, Radbruch A. 2009. Professional memory CD4+ T lymphocytes preferentially reside and rest in the bone marrow. Immunity 30: 721–730. 10.1016/j.immuni.2009.03.015 [DOI] [PubMed] [Google Scholar]
- Trepel F. 1974. Number and distribution of lymphocytes in man. A critical analysis. Klin Wochenschr 52: 511–515. 10.1007/BF01468720 [DOI] [PubMed] [Google Scholar]
- Vu Van D, Beier KC, Pietzke LJ, Al Baz MS, Feist RK, Gurka S, Hamelmann E, Kroczek RA, Hutloff A. 2016. Local T/B cooperation in inflamed tissues is supported by T follicular helper-like cells. Nat Commun 7: 10875. 10.1038/ncomms10875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakim LM, Smith J, Caminschi I, Lahoud MH, Villadangos JA. 2015. Antibody-targeted vaccination to lung dendritic cells generates tissue-resident memory CD8 T cells that are highly protective against influenza virus infection. Mucosal Immunol 8: 1060–1071. 10.1038/mi.2014.133 [DOI] [PubMed] [Google Scholar]
- Walsh DA, Borges da Silva H, Beura LK, Peng C, Hamilton SE, Masopust D, Jameson SC. 2019. The functional requirement for CD69 in establishment of resident memory CD8+ T cells varies with tissue location. J Immunol 203: 946–955. 10.4049/jimmunol.1900052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber JP, Fuhrmann F, Hutloff A. 2012. T-follicular helper cells survive as long-term memory cells. Eur J Immunol 42: 1981–1988. 10.1002/eji.201242540 [DOI] [PubMed] [Google Scholar]
- Westera L, Drylewicz J, den Braber I, Mugwagwa T, van der Maas I, Kwast L, Volman T, van de Weg-Schrijver EH, Bartha I, Spierenburg G, et al. 2013. Closing the gap between T-cell life span estimates from stable isotope-labeling studies in mice and humans. Blood 122: 2205–2212. 10.1182/blood-2013-03-488411 [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. 2007. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27: 670–684. 10.1016/j.immuni.2007.09.006 [DOI] [PubMed] [Google Scholar]
- Wong P, Pamer EG. 2001. Cutting edge: antigen-independent CD8 T cell proliferation. J Immunol 166: 5864–5868. 10.4049/jimmunol.166.10.5864 [DOI] [PubMed] [Google Scholar]
- Youngblood B, Hale JS, Kissick HT, Ahn E, Xu X, Wieland A, Araki K, West EE, Ghoneim HE, Fan Y, et al. 2017. Effector CD8 T cells dedifferentiate into long-lived memory cells. Nature 552: 404–409. 10.1038/nature25144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Bevan MJ. 2013. Transforming growth factor-β signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity 39: 687–696. 10.1016/j.immuni.2013.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. 2005. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat Med 11: 1299–1305. 10.1038/nm1326 [DOI] [PubMed] [Google Scholar]