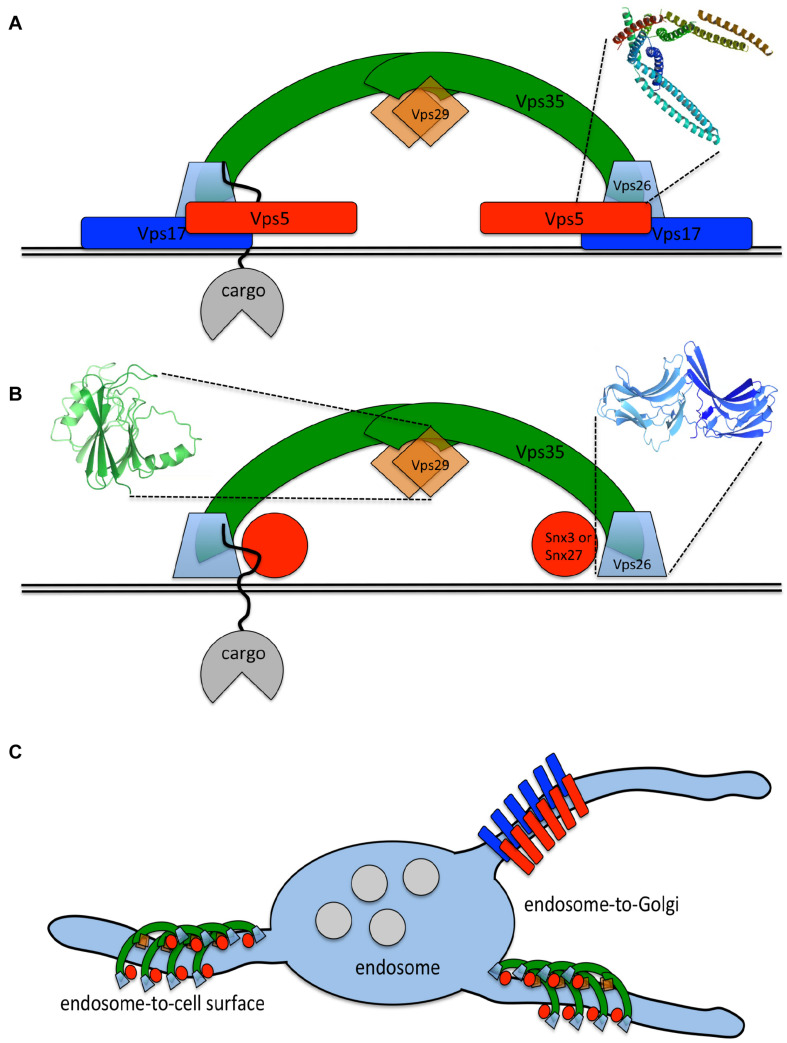
FIGURE 1.
The retromer complex in simple or higher eukaryotes. (A) In a simple eukaryote such as yeast, the retromer complex is a stable heteropentamer that associates with endosomal membranes to sort cargo proteins (e.g., Vps10p) into tubules for endosome-to-Golgi retrieval. The Vps26 protein (along with Vps35p) plays a key role in cargo recognition but Grd19p (SNX3) also contributes to sorting specific cargo. The BAR domain (PDB:4FZS) of SNX-BAR proteins such as Vps5p and Vps17p adopts a curved conformation and can tubulate membranes. (B) In mammals, there is no stable association between the retromer trimer of Vps35-Vps29-Vps26 and the SNX-BAR dimer of SNX1/2-SNX5/6. Both sets of proteins can recognize and sort cargo with similar aromatic motifs being recognized by the retromer trimer (aided by SNX3 and SNX27) and the SNX-BAR dimer. The arrestin-like conformation of VPS26 (in blue, PDB:2R51) may be important in cargo-recognition. Both sets of proteins may also be able to tubulate the membrane, the SNX-BAR dimer via the tubulating activity of the BAR domain and the retromer dimer due to its arch-forming properties that requires VPS35 to fold around the globular VPS29 protein (structure shown in green, PDB:1Z2X). (C) The propensity to tubulate the membrane could enable the retromer trimer and SNX-BAR dimer to generate multiple distinct transport intermediates for trafficking from endosomes to both the Golgi and the cell surface. The destination of the different transport intermediates may depend on the sorting of correct SNAREs into a tubule or association with motor proteins that mediate transport via the microtubule cytoskeleton.