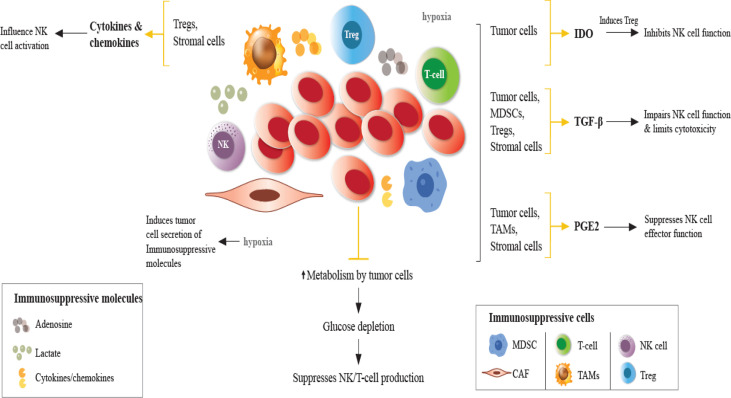
Figure 1.
Impaired NK cell function in the TME. In the TME, immunosuppressive cells and molecules, in the presence of hypoxia and glucose depletion negatively regulate the maturation, proliferation, activation, and effector function of NK cells. Hypoxia induces tumor cells to secrete immunosuppressive molecules, such as TGF-β, IL-10, VEGF, galectins, and CC-chemokine ligands, contributing to the accumulation of TAMs, Tregs, MDSCs, which suppress DCs, T and NK cells. Under hypoxic conditions, NK cells fail to upregulate the expression of activating receptors. Cytokines/chemokines produced by Tregs and stromal cells influence NK cell activity. Tumor cells directly exert immunosuppressive effects on NK cells through the secretion of soluble factors. TGF-β production by tumor cells, Tregs, MDSCs, and other stromal cells impairs NK cell function. IDO induces Treg which inhibits NK cell function. IDO expression induces growth arrest of NK cells and promotes tumor progression. PGE2 production in the TME suppresses the effector function of NK cells. Adenosine secretion by tumor cells and lactate accumulation reduces NK cell cytotoxicity promoting immunosuppression by MDSCs, Tregs and TAMs. TGF-β, transforming growth factor-β; PGE2, prostaglandin E2; IDO, indoleamine 2,3 dioxygenase; NKG2D, natural killer group 2D; MDSCs, myeloid-derived suppressor cells; Tregs, regulatory T cells (Tregs); TAMs, tumor-associated macrophages.