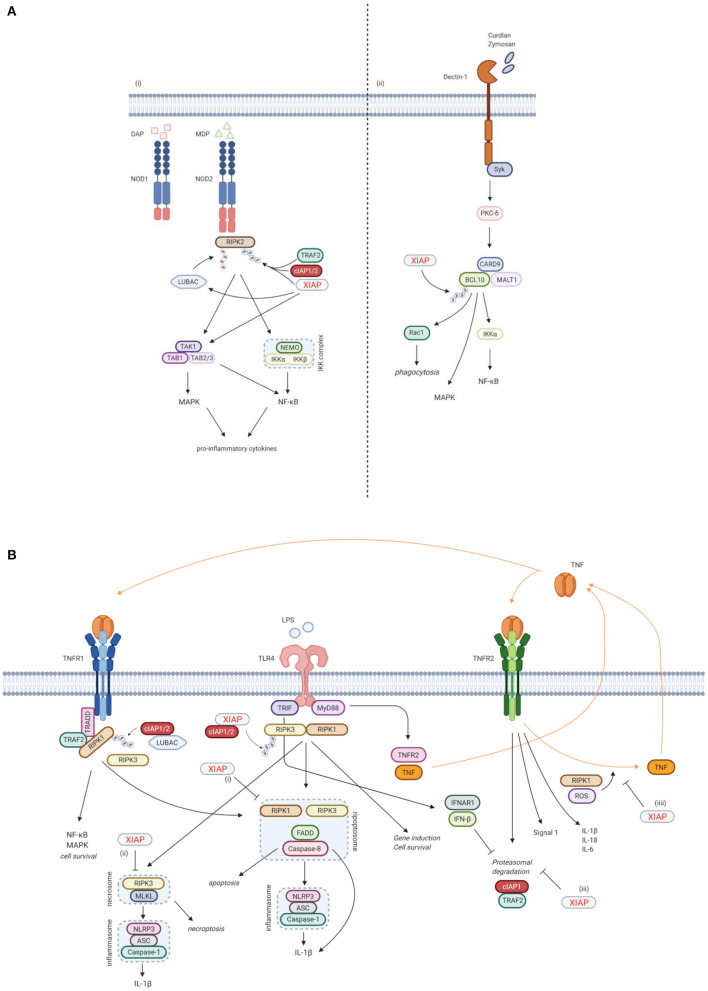
Figure 3.
XIAP plays a key function in various immune pathways. (A) XIAP is required for pattern recognition receptors (PPR) mediated innate immune responses. (i) XIAP is essential for the NOD1/2 induced activation of the NF-κB and MAPK pathways and secretion of pro-inflammatory cytokines and chemokines (40). NOD1 and NOD2 are intracellular PRRs that bind DAP and MDP, respectively (41, 42). Upon ligand binding, XIAP, together with cIAP1/2, ubiquitinates RIPK2, which subsequently acts as a scaffold for the TAK/TAB1 and IKK complexes, leading to activation of the MAPK and NF-κB pathways, respectively (5, 36, 40). The TAK/TAB1 also links to the IKK complex, thereby inducing activation of the NF-κB pathway (5, 36, 43, 44). Upon NOD activation, XIAP also recruits LUBAC, which in turn conjugates linear Ub chains to RIPK2. The concurrent ubiquitination of RIPK2 by XIAP and LUBAC is necessary for efficient activation of the canonical IKK for NF-κB activation (36, 44, 45). (ii) XIAP is necessary in Dectin-1 signalling. Dectin-1 is a transmembrane PRR that detects β-glucan. Upon Dectin-1 stimulation, XIAP binds and ubiquitinates BCL-10, which is essential for the activation of the NF-κB and MAPK pathways and cytokine production. BCL-10 is also involved in Rac1-dependent phagocytosis, which again relies on ubiquitination of BCL-10 by XIAP (28). (B) XIAP regulates the activation of inflammasomes via TLR/TNFR signalling. (i) Following TNFR1 and TLR signalling, RIPK3 can recruit RIKP1 to activate caspase-8 (46, 47). In this process, XIAP has an inhibitory effect on the ripopotosome by controlling the ubiquitination of RIKP1 in a RIPK3-dependent manner (6, 48). (ii) XIAP inhibits MLKL necroptotic signalling, which is promoted by RIPK3 in the absence of caspase-8 (48). (iii) TLR-MyD88 signalling causes the proteasomal degradation of cIAP1 and its adaptor TRAF2 by inducing TNF and TNFR2 signalling. This proteasomal degradation is inhibited by TLR-TRIF induced IFN-β. Loss of XIAP promotes LPS-induced cIAP1 degradation. Subsequently, cIAP1 loss in the absence of XIAP promotes TLR-induced RIPK3 caspase-8 and IL-1β activity, eventually leading to IL-1β maturation, caspase-8 cleavage and enhanced cell death (49). (iiii) TNFR2 activation acts as a signal 1 for priming the canonical inflammasome and induces expression of pro-inflammatory cytokines. XIAP loss and TNFR1 signalling play a role as signal 2 for activation of the inflammasome. This is mediated by RIPK1 and ROS production. In the absence of XIAP, TNFR2 stimulation leads to TNF production, followed by TNFR1 mediated cell death (50). Created with BioRender.com.