FIGURE 1.
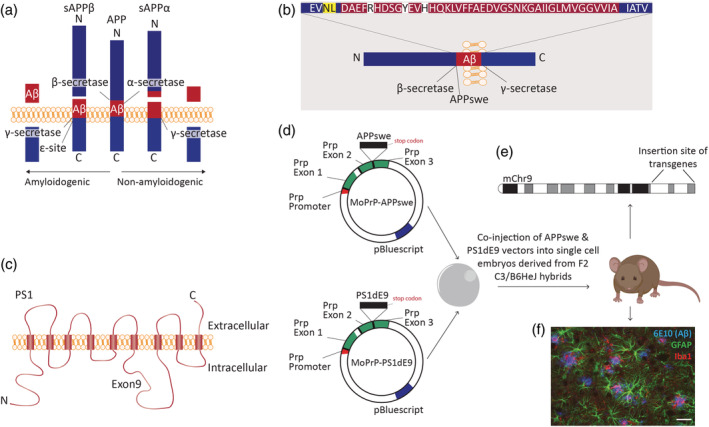
An overview of the amyloidogenic and non‐amyloidogenic pathway, the generation of the APPswePS1dE9 mouse model, and gliosis. (a) Alternative processing of APP by α‐, β‐, and/or γ‐secretases resulting in amyloidogenic and non‐amyloidogenic cleavage. (b) Humanized mouse APP sequence (the white blocks indicate the human‐specific amino acids that were introduced in the mouse sequence), containing the Swedish mutation (yellow block). (c) PS1 is a transmembrane protein and part of the γ‐secretase complex. The PS1dE9 mutation results in the deletion of exon 9. (d) The APPswe and PS1dE9 transgenes were integrated in MoPrP vectors between exon 2 and 3; the coding sequence of PrP was completely removed, as described in Borchelt et al. (1996) and Jankowsky et al. (2001). The two vectors were co‐injected into a single cell embryo derived from F2 hybrids of C57BL/6J and C3H/HeJ mice: C3/B6HeJ mice. This resulted in the generation of double transgenic APPswePS1dE9 mice, in which the expression of both the APP and the PS1 gene is driven by the mouse prion protein promoter. (e) Integration of the transgenes occurred at chromosome 9 between the Arpp21 and Pdcd6ip genes (Jackson et al., 2015). (f) From the age of 6 months, reactive astrocytes (GFAP, green) and activated microglia (Iba1, red) surrounding Aβ plaques (6E10, blue) were detected, scale bar: 50 μm, adjusted from original picture in Orre et al. (2014). Aβ, amyloid‐beta; APP, amyloid precursor protein; ε‐site, epsilon cleavage site; GFAP, glial fibrillary acidic protein; Iba1, ionized calcium‐binding adapter molecule 1; MoPrP, modified prion protein; PrP, prion protein; PS1, presenilin 1; sAPPα, soluble amyloid precursor protein‐α; sAPPβ, soluble amyloid precursor protein‐β. Based on Jackson et al. (2015) and Jankowsky et al. (2001, 2007). Scientific illustration toolkits from Motifolio were used to generate parts of this figure
