Figure 4.
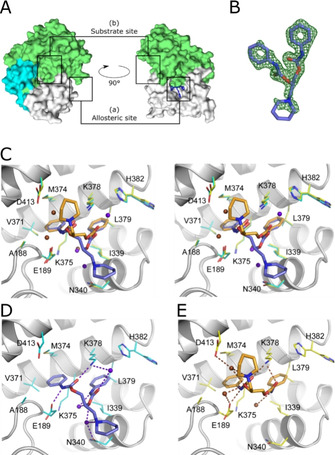
Crystal structures of SMYD3 in complex with diperodon. A) SMYD3 with diperodon (blue sticks) bound to the allosteric site (a), distinctly separated from the substrate binding site (b). The surface is colored to visualize the three main domains of SMYD3: SET and MYND domains (green), post‐SET domain (cyan), C‐terminal domain (white). B) (F 0−F c) difference density for (S)‐diperodon (green mesh, contoured at 3 σ). C) Visualization of the binding modes of (S)‐ and (R)‐diperodon in the allosteric site (stereoview, PDB IDs: 6Z2R and 6YUH). The ligands are shown as thick sticks colored by atom, with the carbon atoms of the S enantiomer in steel‐blue and those of the R enantiomer in orange. Amino acid residues within a 4 Å radius of either ligand are shown as thin sticks with carbon atoms in cyan and yellow for complexes with the S and R enantiomers, respectively. Water molecules interacting with the S and R enantiomers are colored brown and blue/purple, respectively. Details of the hydrogen‐bonding interactions of D) (S)‐diperodon (ligand in steel blue) and E) (R)‐diperodon (ligand in orange). Ligands and interacting SMYD3 residues are depicted as in (B), hydrogen bonds are shown as dashed lines.
