Figure 1.
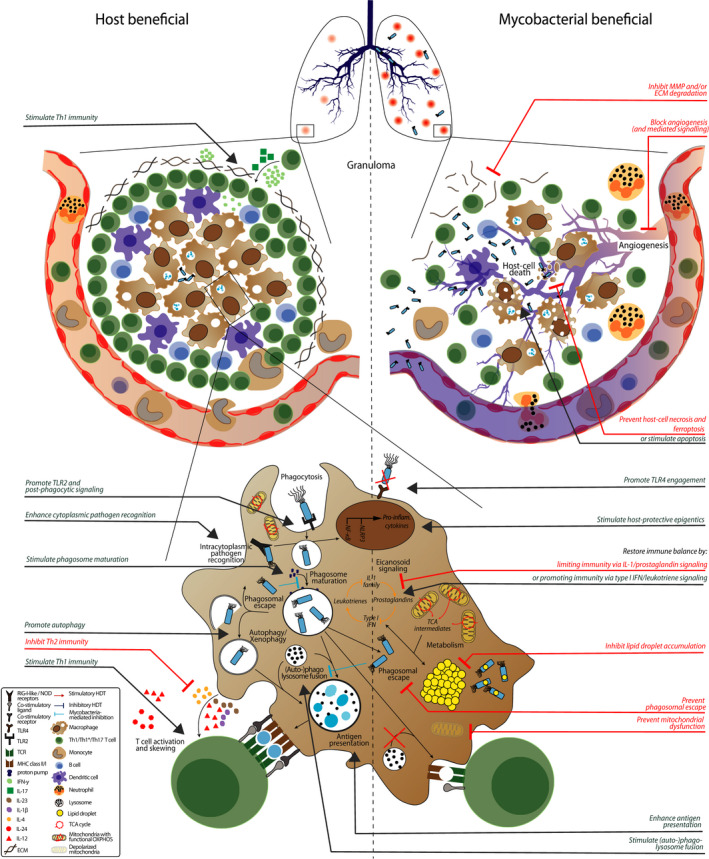
Host‐pathogen interactions and potential host‐directed therapies (HDT). Granulomas are characteristic for tuberculosis and mycobacterial infections in general. Pathologic granulomas are poorly vascularized due to ineffective angiogenesis, leading to hypoxia and concomitant host‐cell necrosis and bacterial dissemination. Blocking angiogenesis, preventing host‐cell necrosis (or stimulating apoptosis) or inhibiting extracellular matrix (ECM) degradation improves granuloma structure and concomitant disease outcome. Macrophages, key cells in the anti‐mycobacterial response, initiate phagocytosis after toll‐like receptor (TLR) recognition, which is prevented and/or modulated by mycobacteria. Promoting TLR4 engagement, TLR2 signaling and post‐phagocytic signaling via receptor tyrosine kinase are all potential targets for HDT to improve host immunity during mycobacterial infection. After internalization, mycobacteria are located to phagosomes that slowly mature and ultimately fuse with lysosomes, which are all inhibited by mycobacteria. Alternatively, mycobacteria escape to the cytosol where they can be recognized by cytoplasmic pathogen recognition receptor (PRR) and “recaptured” using autophagy, which again is inhibited by mycobacteria. HDTs that (1) prevent phagosomal escape, (2) alleviate blockage of (auto‐)phagosome maturation, (3) promote autophagy and/or (4) stimulate (auto‐)phagolysosome fusion all enhance mycobacterial killing. HDT that enhance cytoplasmic recognition of mycobacteria also improve the anti‐mycobacterial immune response. Mycobacteria that remain in the cytosol impair host metabolic pathways by stimulating tricarboxylic acid (TCA) cycle intermediates from mitochondria to be expelled into the cytosol to form lipid droplets and induce mitochondrial membrane depolarization. HDTs that (1) impair lipid droplet accumulation, (2) prevent mitochondrial membrane depolarization, and/or (3) stimulate TCA cycle intermediates being allocated in eicosanoid signaling maintain macrophage functionality which leads to better mycobacterial control. Finally, mycobacteria prevent the host from mounting an effective adaptive immune response by inhibiting antigen presentation and impairing T‐cell skewing. HDTs that promote adaptive immunity by enhancing antigen presentation, stimulating Th1 skewing or inhibiting Th2/Treg immunity all improve disease outcome. Compounds that can correct the above processes are represented in red for inhibitory/blocking therapies and in green for stimulatory therapies and summarized in Table 1, ordered per physiological process
