FIGURE 1.
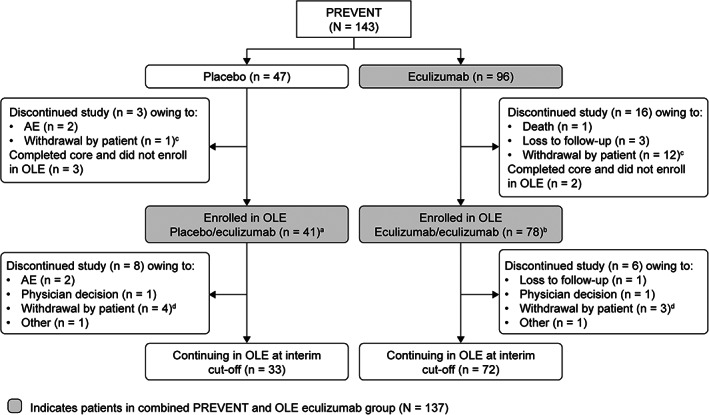
Patient disposition through July 31, 2019. aFifteen patients reached the end of PREVENT relapse‐free, and 26 patients entered the OLE following a relapse as determined by the treating physician. bSixty‐four patients reached the end of PREVENT relapse‐free, and 14 patients entered the OLE following a relapse as determined by the treating physician. cThe 13 patients who withdrew consent (patient decision) during PREVENT included 1 patient in the placebo group who did not wish to continue taking an investigational product, and 12 patients in the eculizumab group who withdrew for the following reasons: a change in life situation or moving to a different area (7 patients); unknown reasons (3 patients); ongoing AEs not related to study drug and difficult venous access (1 patient); and clinical trial fatigue (1 patient). dThe 7 patients who withdrew consent (patient decision) during the OLE included 4 in the placebo/eculizumab group for the following reasons: a change in life situation or moving to a different area (2 patients); to receive treatment with traditional Chinese medicine (1 patient); and unwillingness to continue study visits following an SAE at enrollment and 1 month on study (1 patient). Three patients in the eculizumab/eculizumab group withdrew for the following reasons: study dosing schedule and an AE described as urticaria (1 patient); inability to travel to the study site owing to back pain (1 patient); and a change in life situation (1 patient). AE = adverse event; OLE = open‐label extension; SAE = serious adverse event.
