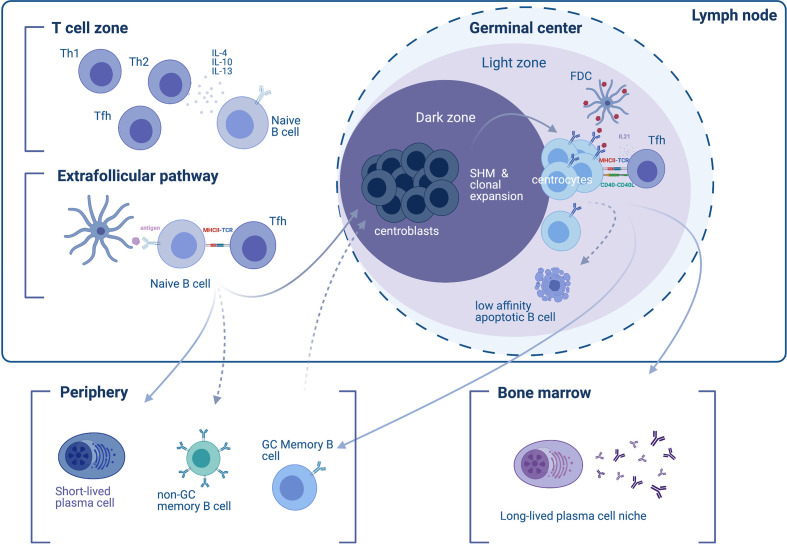
Figure 1.
Differentiation of B cells into short- and long-lived antibody-secreting cells. In the initial phase of the immune response to a T cell-dependent antigen, responding naïve B cells appear in the T cell zone of the lymph node (upper left), where their development and differentiation is facilitated by T cell-secreted cytokines. T helper type 2 (Th2) type cytokine secretion, such as IL-4, -10, and -13 favors the induction of an IgG4 response. B cells enter the extrafollicular pathway and undergo B cell receptor (BCR) activation by encountering antigens on follicular dendritic cells (FDCs), which they then present to T follicular helper (Tfh) cells through MHC-II. The extrafollicular pathway gives rise to (i) short-lived plasmablasts (SLPBs) that enter the periphery, and (ii) germinal center (GC)-independent memory B cells. In a second phase, activated B cells enter the GC dark zone, where they mutate (a process called somatic hypermutation) and clonally expand (therefore termed centroblasts). B cells cycle between the dark and the light zone (where they are termed centrocytes). The dynamic cycle of the GC allows centrocytes that entered the light zone to be chosen based on the affinity of their BCRs to the antigen. Low-affinity B cells that are not presenting antigen on their BCRs will eventually become apoptotic and die. B cells that do present the antigen receive help from Tfh through CD40L and IL21 survival signals. The end-products of the GC reaction are (i) memory B cells, and (ii) long-lived plasma cells (LLPCs). GC memory B cells will enter the periphery and re-enter the GC upon BCR stimulation. LLPCs exit the GC and find a survival niche, typically the bone marrow.