Fig. 7.
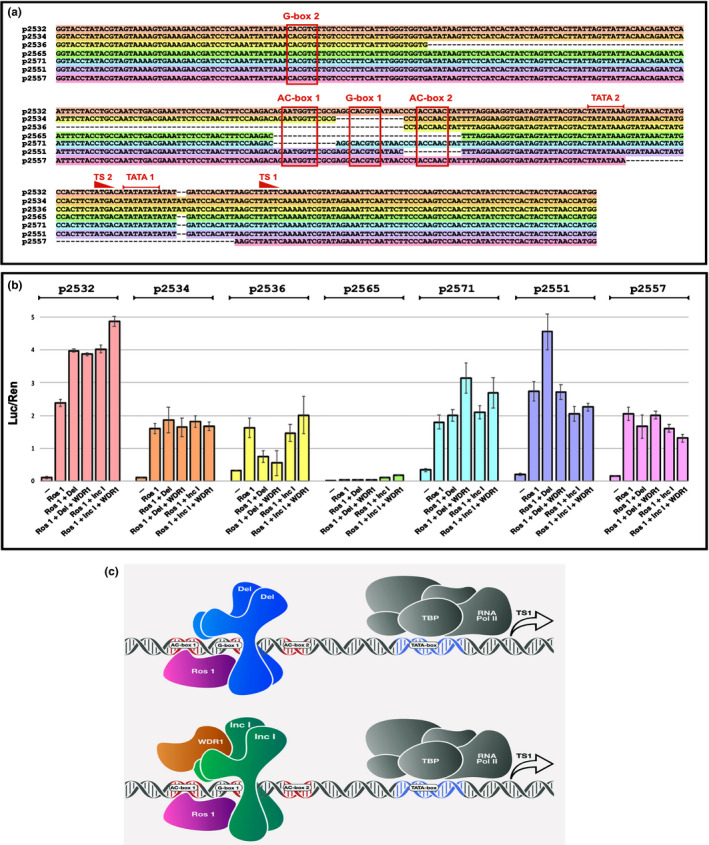
Assessment of responsiveness of the DFR promoter from A. majus to components of the MBW complex from A. majus using dual luciferase assays in N. benthamiana. (a) Structure of the wild‐type promoter of the DFR gene from A. majus (p2532) showing the gene proximal transcriptional start site (TS1) and associated TATA box (TATA 1) and the gene distal transcriptional start site (TS2) and its associated TATA box (TATA 2) (Coen et al., 1986).G‐box 1 is shown flanked on its gene distal side by AC‐box 1 and on its gene proximal side by AC‐box 2. It should be noted that AC‐box 1 reads 5′ to 3′ on the lower DNA strand whereas AC‐box 2 reads 5′ to 3′ on the upper strand. The location of a second G‐box (G‐box 2) is also shown. Below the sequence of the WT promoter (p2532; highlighted in pink) the deletions assayed using the dual luciferase assay are shown, coloured in accordance with the bars detailing the effects of these deletions on luciferase reporter activity shown in panel b). (b) Activation of luciferase by MBW components on wild‐type DFR promoter (pink) and promoters carrying deletions of the key upstream activating sequences (UAS) detailed in panel (a). All values relate luciferase to Renilla luminescence and are the results from at least five independent assays. Error bars show SEMs. (c) Model illustrating the predominant binding of MBW components to the wild‐type DFR promoter from A. majus. The bHLH proteins Del and Inc I bind to G‐box 1 probably as a dimer. The N‐termini of Del and Inc I interact with the surface exposed region of R3 of the Myb DNA binding domain of Ros1 carrying the bHLH interaction signature motif (Zimmermann et al., 2004). TBP = TATA Binding Protein For Inc I the additional binding by WDR1 is necessary for full activity.
