Table 2.
Catalytic systems, major products, maximum FEs, and mechanisms of Mn complexes in electrochemical CO2 reduction (n.a.=not available, prop.=proposal, comp.=computational investigation, exp.=experimental evidence).
|
Entry |
Cat. system |
Substitution |
Major product |
Max. FE (%) |
Mechanism |
Basis |
Method |
Ref. |
|---|---|---|---|---|---|---|---|---|
|
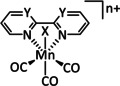
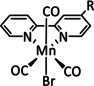
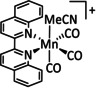
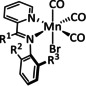
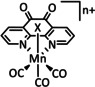
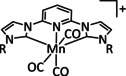
1a |
|
n=0, 1 X=CN, CO, MeCN Y=CH, N |
CO/H2O |
98 [89] |
ETM |
exp. comp. |
[86, 88–91] |
|
|
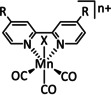
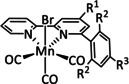
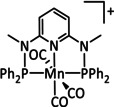
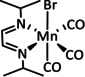
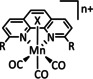
1b |
|
n=0, 1 R=H, Me, Et, tBu, Ph, Bn, CN, CO2H, CO2C3H6C4H5N, CF3, NMe2, OH, OMe, SMe X=MeCN, Br |
CO/H2O H2 |
100±15 [80] 45 [92] |
ETM |
exp. comp. |
DFT [93] |
[79–80, 92–94] |
|
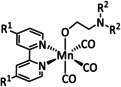
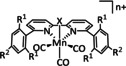
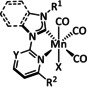
1c |
|
R1=H, OMe, Br R2=C2H4OH |
n.a. |
n.a. |
ETM |
exp. comp. |
IR, NMR DFT |
|
|
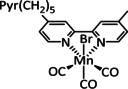
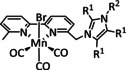
1d |
|
– |
CO/H2O HCO2H H2 |
34±4 8±2 59±8 |
low c cat: ETH high c cat: ETM |
exp. |
IR‐, UV/Vis‐SEC |
|
|
1e |
|
R=C6H4NH2, SMe |
CO/H2O |
100±5 [94a] |
ETM |
prop. [97] |
n.a. |
[94a, 97, 98] |
|
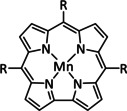
1f |
|
R1=H, Ph R2=H, CH2NEt2, OH, OMe R3=H, OH, F |
CO HCO2H |
90 [99] 63 [100] |
no H+: ETM H+: ETH ETM |
NMR, IR‐SEC DFT |
[62a, 99–102] |
|
|
1g |
|
n=0, 1 R1=H, Me, Et, CH2NHEt, CH2NEt2, CH2‐morpholine, CH2OH, CHO, CO2H, NH2, OH,OMe, F R2=H, Me, CH2NEt2, OH X=MeCN, OTf, Br |
CO/CO3 2−[64, 103] CO/H2O [103] HCO2H |
98±3 [64] n.a. 90 [100] |
ETM ETH |
exp. exp. [100] comp. [100] |
IR‐SEC, [64] (PR‐) TRIR [104] DFT IR‐SEC, NMR DFT |
[64, 81, 100, 103, 104] |
|
1h |
|
R1=H, Me R2=Me, tBu |
CO/H2O |
78 |
ETM |
comp. |
DFT |
|
|
1i |
|
– |
CO/H2O |
98 |
ETM |
comp. |
DFT |
|
|
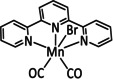
2a |
|
– |
CO/CO3 2− |
96 |
n.a. |
n.a. |
n.a. |
|
|
2b |
|
– |
CO/CO3 2− H2 |
96 (CO+H2) |
n.a. |
n.a. |
n.a. |
|
|
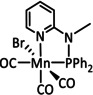
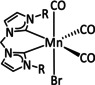
3 |
|
R1=Me, Et R2=H, C6H4OH X=CN, SCN, Br, I Y=CH, N |
CO/H2O H2 |
73 [106] 53 [107] |
ETM |
DFT |
[106–108, 109] |
|
|
4 |
|
R1=H, Me R2=H, iPr, tBu R3=H, iPr |
CO/CO3 2− |
60 |
ETM |
exp. comp. |
IR‐, UV/Vis‐SEC DFT |
|
|
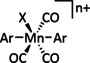
5 |
|
– |
CO/H2O |
n.a. |
ETM |
exp. |
IR‐, UV/Vis‐SEC |
|
|
6 |
|
Ar=CN(2,6‐(2,6‐(iPr)2C6H3)2C6H3) n=0, 1 X=THF, Cl, Br, I |
CO/CO3 2− |
n.a. |
ETM |
exp. |
IR‐SEC |
|
|
7 |
|
– |
CO/H2O |
129[a] |
n.a. |
n.a. |
n.a. |
|
|
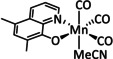
8a |
|
n=0, 1 X=MeCN, Br |
CO/H2O |
100 |
ETM |
exp. |
EPR, IR‐, UV/Vis‐SEC |
|
|
8b |
|
n=0, 1 R=H, Me, C6H4CH2NEt2 X=MeCN, Br |
CO/H2O HCO2H |
62 [91] 70 [100] |
ETM (CO) ETH (HCO2H) |
exp. |
NMR, IR‐SEC [100] DFT |
[91, 94c, 100] |
|
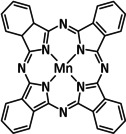
9 |
|
R=Me, Mes |
CO/CO3 2− CO/H2O |
95 [114] 98 [109b] |
ETM |
exp. [109b] comp. [109b] |
IR‐, [114] UV/Vis‐SEC DFT |
|
|
10 |
|
– |
CO/H2O |
88 |
ETM |
exp. comp. |
EPR‐, IR‐SEC, PR‐TRIR DFT |
|
|
11 |
|
R=Me, Bn |
CO/CO3 2− CO/H2O |
93 [116] 87±3 [117] |
ETM |
DFT |
||
|
12 |
|
R=C6F4‐S‐(PEG7)‐OMe |
CH3OH CH3CO2 − |
23 63 |
ETM |
exp. comp. |
EAS,[b] GC‐MS, IL, IR‐, UV/Vis‐SEC, NMR DFT |
|
|
13 |
|
– |
HCO2 − H2 |
26 77 |
n.a. |
n.a. |
n.a. |
[a] Likely caused by loss of carbonyl ligand. [b] Electronic absorption spectroscopy.