Table 3.
Catalytic systems, major products, maximum FEs, and mechanisms of Re complexes in electrochemical CO2 reduction (n.a.=not available, prop.=proposal, comp.=computational investigation, exp.=experimental evidence).
|
Entry |
Cat. system |
Substitution |
Major product |
Max. FE (%) |
Mechanism |
Basis |
Method |
Ref. |
|---|---|---|---|---|---|---|---|---|
|
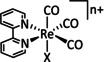
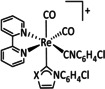
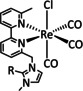
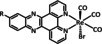
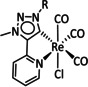
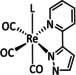
1a |
|
n=0, 1 X=H, MeCN, CO, HCO2, HCO3, CH3C(O), OMe, THF, OTf, PPh3, P(OEt)3, Cl, Br |
CO/CO3 2− CO/H2O HCO2 − H2 |
98 [123] n.a. [125] 74 [126] |
ETM |
exp. |
EPR, [128] IR‐SEC,[ 125 , 129 ] Raman [130] DFT |
[26, 60a, 63, 122–131] |
|
|
|
|
|
|
|
|
|
|
|
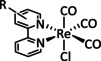
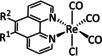
1b |
|
R=vinyl, ethynyl, C6H4NH2, norbornenyl derivatives, CH2NHCOCH3/peptide resins, 4‐piperidinyl‐1,8‐naphthalimide, NHCSNH‐C6H4CF3, SMe, thiophene, 2,2′:5′,2′′‐terthiophene, 3′‐ethynyl 2,2′:5′,2′′‐terthiophene |
CO/H2O |
100 [98] |
ETM |
exp. [132] |
IR‐SEC |
[94a, 98, 132, 133] |
|
|
|
|
|
|
|
|
|
|
|
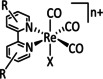
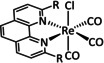
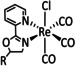
1c |
|
n=0, 1 R=Me, tBu, bisphenylethynyl, CH2NHCOCH3, tyrosyl derivative, CH2NEt2, CH2OH, CN, CO2H, CF3, NH2, NHMe, NMe2, OH, OMe, Si(Ph)4 X=H2O, Cl |
CO/CO3 2−[134] CO/H2O HCO2H |
100 [94f] 71 [135] 12 [136] |
ETM |
exp. comp. [130] |
EPR, [130] IR‐SEC, [87a] Raman [130] DFT |
[82, 94f, 130, 133a, 134–137] |
|
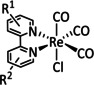
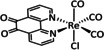
1d |
|
R1=H, C2H3 R2=Me, NHMe, NMe2, CF3 |
CO/CO3 2− CO/H2O |
92 [138] 73 [94f] |
n.a. |
n.a. |
n.a. |
|
|
1e |
|
X=O, S |
CO/CO3 2‐ |
90 |
n.a. |
n.a. |
n.a. |
|
|
1f |
|
– |
CO/H2O |
89 |
ETM |
exp. comp. |
IR, UV/Vis‐SEC DFT |
|
|
1g |
|
R=C2H4OH |
CO HCO2H |
95 [141] 27 [141] |
ETM (CO) ETH (HCO2H) |
exp. comp. |
DFT [95] |
|
|
1h |
|
R1=Ph, C6H4OH R2=Ph, phenyl‐2,6‐ diol, phenyl‐3,4,5‐triol |
CO/CO3 2− |
100 |
ETM |
prop. |
n.a. |
|
|
1i |
|
R=H, Me |
CO |
73 |
ETM |
prop. |
n.a. |
|
|
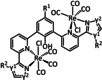
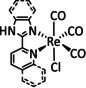
2a |
|
R1=H, NH2, 4‐piperidinyl‐ 1,8‐naphthalimide R2=H, NH2 R1∩R2=nanographene |
CO/H2O |
96 [144] |
ETM |
comp. [145] |
DFT |
|
|
2b |
|
R=H, Me, Mes, 4‐MeOC6H4, 2,6‐(MeO)2C6H3, 3,5‐(MeO)2C6H3, 2,4,6‐(MeO)3C6H2, 3,4,5‐(MeO)3C6H2 |
CO/H2O |
84 [146] |
ETM |
prop. |
n.a. |
|
|
2c |
|
– |
CO/CO3 2− |
n.a. |
ETM |
prop. |
n.a. |
|
|
2d |
|
R=H, tBu, CF3, NO2 |
CO |
53 |
n.a. |
n.a. |
n.a. |
|
|
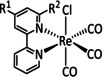
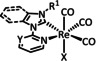
3a |
|
R1=Me, p‐C6H4R2, (m‐CF3)2C6H3 R2=CN, CF3, NO2 X=Cl, Br Y=CH, N |
CO/H2O |
92 [84] |
ETM |
prop. [84] |
n.a. |
|
|
3b |
|
R1=tBu, pyrenyl R2=H, Me Y1=C, N Y2=CH, NH |
CO/H2O |
85 [151] |
n.a. |
n.a. |
n.a. |
|
|
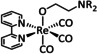
4a |
|
R1=C12H25, tolyl, C6H2(tBu)3, C3H6OH R2=H, NO2, OMe X=Cl, Br |
CO/H2O |
92 |
n.a. |
n.a. |
n.a. |
|
|
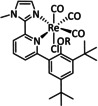
4b |
|
R=2,6‐(iPr)2C6H3, CH2C6H5, CH2C6F5 |
CO/H2O |
99 |
ETM |
exp. |
IR‐SEC |
|
|
5 |
|
R=H, Me, Ph |
CO/H2O |
61 |
ETM |
comp. |
DFT |
|
|
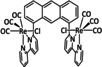
6a |
|
– |
n.a. |
n.a. |
n.a. |
n.a. |
n.a. |
|
|
6b |
|
R=H, Me |
CO |
105±5 |
n.a. |
n.a. |
n.a. |
|
|
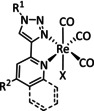
7 |
|
L=pyrazole, 3,5‐dimethylpyrazole, indazole, 3‐(2‐pyridyl)pyrazole |
CO |
89 |
ETM |
prop |
n.a. |
|
|
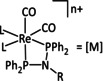
8 |
|
L=CO, Cl L∩L=bpy, phen n=0, 1 R=H, Ph, tolyl,C6H4Br, C6H4‐[M] |
CO/H2O |
94 |
ETM |
prop. |
n.a. |