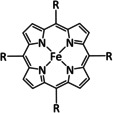
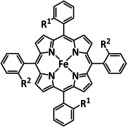
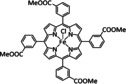
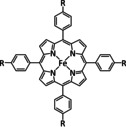
Table 4.
Catalytic systems, major products, maximum FEs, and mechanisms of Fe complexes in electrochemical CO2 reduction (n.a.=not available, prop.=proposal, comp.=computational investigation, exp.=experimental evidence).
|
Entry |
Cat. system |
Substitution |
Major product |
Max. FE (%) |
Mechanism |
Basis |
Method |
Ref. |
|---|---|---|---|---|---|---|---|---|
|
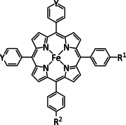
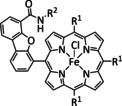
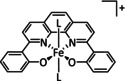
1a |
|
R=Ph, C6F5, pyren‐1‐yl, meso‐thien‐2‐yl, meso‐5‐methylthien‐2‐yl |
CO/H2O HCO2 − |
100 [160d] 72[a] [164] |
ETM |
DFT |
FeII: FeIII:[b] |
|
|
1b |
|
R1=R2=CO2Me, NHCOtBu, NHCOC6H4CH2MeIm+, NMe3 +, trFc2, trCO2Me, tr‐4‐tBu R1∩R1/R2∩R2=NHCO(CH2)10CONH, NHCO(CH2)10ImCONH |
CO/H2O |
100 [160h] |
ETM |
DFT |
FeII: [166f] FeIII: |
|
|
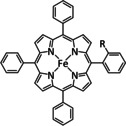
1c |
|
– |
CO/H2O |
65 |
ETM |
comp. |
DFT |
|
|
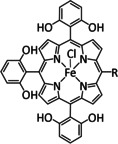
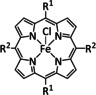
1d |
|
R=Ph, pyren‐1‐yl, CO2Me, NMe3 +, SO3 − |
CO/H2O H2 |
100 [160e] 84 [160f] |
ETM |
comp. [160f] |
DFT |
FeII: [167] |
|
1e |
|
R1=CH2CONHC6H3(CF3)2, NHCOCH2C6H3(CF3)2, NHCONH‐Fe‐TPP, OMe R2=H, NH2, OMe Y=CH, N |
CO/H2O CH4 |
90 [169] 41 [163] |
ETM |
comp. [167] |
DFT |
|
|
1f |
|
R=OH, OMe |
CO/H2O |
94 [160c] |
ETM |
prop. |
n.a. |
FeII: [160g] |
|
1g |
|
R=propylpyrene |
CO/H2O |
97 |
n.a. |
n.a. |
n.a. |
|
|
1h |
|
R=CH2CONHC6H3(CF3)2, NHCOCH2C6H3(CF3)2, OH |
CO/H2O |
96 [172] |
ETM |
comp. [167] |
DFT |
FeII: [167] FeIII: [172] |
|
1i |
|
R1=3,4,5‐trimethoxyphenyl R2=CNHNH2, C6H4OH, C6H4SO3H |
CO/H2O |
96 |
ETM |
comp. |
DFT |
|
|
1j |
|
R1=3,4,5‐trimethoxyphenyl R2=3,4,5‐trimethoxyphenyl |
CO/H2O |
100 |
ETM |
prop. |
n.a. |
|
|
| ||||||||
|
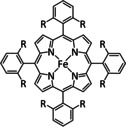
1k |
|
R=Ph, Me3C6H2, C6F5, 2,6‐Cl2C6H3, 2,6‐F2C6H3 |
CO/H2O |
92 [175] |
ETM |
prop. [175] |
n.a. |
|
|
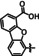
2a |
|
L=MeIm |
CO/H2O HCO2 − C2O4 2− |
42 74 11 |
ETM (CO/C2O4 2−) ETH (HCO2 −) |
exp. prop. |
IL,[c] IR‐SEC |
|
|
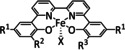
2b |
|
R1=Me, tBu R2=tBu, OH, OMe R3=tBu, OMe X=–, Cl |
HCO2H H2 |
85 [178] 60 [178] |
ETH |
exp. [179] comp. [179] |
IR‐SEC |
|
|
DFT | ||||||||
|
|
|
|
|
|
|
|
|
|
|
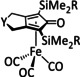
3 |
[Fe4Y(CO)11(L)]n‐ |
L=CO, PPh3, PPh2(CH2)2OH, PPh2C6H4tr n=1, 2 Y=C, N |
HCO2 − H2 |
96±2 [180] 96±6 [181] |
ETH |
exp. [180] |
IL, IR‐SEC, XRD |
|
|
4 |
|
– |
HCO2 − |
80 |
ETM |
comp. |
DFT |
|
|
5 |
[Fe(N∩N)3]2+ |
N∩N=bpy, phen |
CO/CO3 2− |
n.a. |
outer sphere |
exp. comp. |
UV/Vis‐SEC DFT |
|
|
|
|
|
|
|
|
|
|
|
|
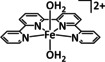
6 |
[Fe(tpy)2]2+ |
– |
CO/CO3 2− |
n.a. |
outer sphere |
exp. comp. |
UV/Vis‐SEC DFT |
|
|
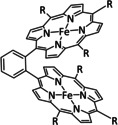
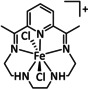
7 |
|
R=Me, tBu Y=CH2, C2H4, O |
CO/H2O |
98 [161b] |
ETM |
exp. comp. |
chem. isol., IR‐SEC DFT |
|
|
8 |
|
– |
CO/H2O |
48 |
ETM |
exp. comp. |
IR‐, UV/Vis‐SEC DFT |
|
|
9 |
|
L=H2O, MeCN, CF3SO3 − n=0, 1, 2 R=H, NHEt, NEt2, OH, OMe |
CO/H2O |
81 [185] |
ETM |
comp. [186] |
DFT |
|
|
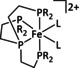
10 |
[Fe(N2)(dmpe)2] |
– |
CO/CO3 2− |
n.a. |
ETM |
exp. |
IL, NMR, XRD |
|
|
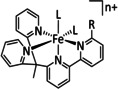
11 |
|
L=MeCN R=Ph |
HCO2 − MeOH[d] |
97 69 |
ETH |
exp. |
IL, NMR |
[a] After addition of Et3N. [b] The metal is coordinated by an axial halide ligand. [c] IL=isotopic labeling. [d] After addition of NHEt2.