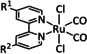
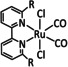
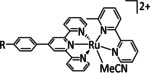
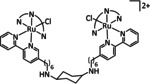
Table 5.
Catalytic systems, major products, maximum FEs, and mechanisms of Ru and Os complexes in electrochemical CO2 reduction (n.a.=not available, prop.=proposal, comp.=computational investigation, exp.=experimental evidence).
|
Entry |
Cat. system |
Substitution |
Major product |
Max. FE (%) |
Mechanism |
Basis |
Method |
Ref. |
|---|---|---|---|---|---|---|---|---|
|
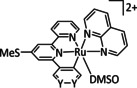
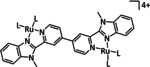
1a |
[Ru(bpy)(CO)mLo]n+ |
L=Cp, CO2Me, py‐CO2 −, qui, Cl m=0, 1, 2; n=0, 1, 2; o=1, 2 |
CO/CO3 2− CO/H2O H3CCOCH3 [a] |
97 [190] n.a. 70 [194] |
ETM |
comp. [197] |
DFT |
[189, 190, 194, 197, 198] |
|
1b |
|
R1=Me, tBu, pyrrol‐1‐ylethyl, CO2‐iPr, pyrrolylpropyl carbonate R2=Me, tBu, CO2‐iPr, pyrrolylpropyl carbonate |
CO/H2O HCO2H |
100 [189b] 97 [94b] |
ETM (CO) [199] ETH (HCO2H) [199] |
prop. |
n.a. |
[94b, 189b, 198a] |
|
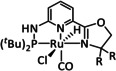
1c |
|
R=Mes, CH2NEt2 |
CO/H2O HCO2H |
95 [67] 9 [136] |
ETH |
exp. |
IR‐SEC |
|
|
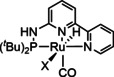
1d |
[Ru(di‐R‐bpy)2(CO)mLo]n+ |
L=H, EtOH, qui, 1,8‐napy, Cl m=0, 1, 2; n=0, 1, 2; o=0, 1, 2 R=H, CO2H |
CO/CO3 2− CO/H2O HCO2 − H3CCOCH3 DMF[b] H2 |
78 [195] 88 [200] 84 [201] 16 [195] 21 [196] 51 [201] |
ETM ETH |
exp. [196] comp. [202] exp. [203] |
IR, NMR [196] DFT IR, NMR, UV/Vis |
[195, 196, 200–204] |
|
|
|
|
|
|
|
|
|
|
|
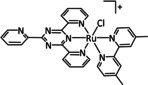
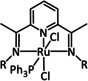
2a |
[Ru(tri‐R1‐tpy)L]n+ |
L=H2O, CH3CN, CO, di‐R2‐bpy, phen‐CO2 −, dmphen,[c] Mebim‐py,[d] pbn, 8‐(diphenylphosphanyl)‐qui, Cl n=1, 2 R1=H, tBu, NO2 R2=H, Me, tBu, OMe |
CO/CO3 2− CO/H2O HCO2 − |
95 [205] 85 [206] 42 [191] |
ETM |
comp. [207] |
DFT |
[191, 192, 205–208] |
|
|
|
|
|
|
|
|
|
|
|
2b |
[Ru(4‘‐R‐tpy)(L)(X)]n+ |
L=bpy, tpy n=1, 2 R=H, 4‐(tert‐butyl‐phenyl)‐1H‐1,2,3‐triazol‐4‐yl X= –, Cl |
CO/H2O HCO2 − H2 |
38 10 33 |
ETM |
prop. |
n.a. |
|
|
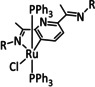
2c |
|
R=PO3H2, PO3Et2 |
CO/H2O |
63 |
ETM |
prop. |
n.a. |
|
|
2d |
|
N∩N∩N=tpy |
CO/CO3 2− |
22 |
n.a. |
n.a. |
n.a. |
|
|
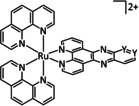
2e |
|
Y=CH, NMe |
CO/CO3 2− |
35 |
ETM |
exp. |
IR‐SEC |
|
|
3 |
|
– |
CO/CO3 2− |
97 |
ETM |
comp. |
DFT |
|
|
4a |
|
Y=CH, N |
HCO2 − H2CO MeOH |
n.a. |
ETH |
exp. |
IR‐SEC |
|
|
4b |
|
– |
CO/CO3 2− |
96 |
ETM |
comp. |
DFT |
|
|
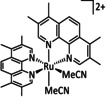
5 |
|
L=bpy |
HCO2 − C2O4 2− |
90 70 |
ETM |
exp. |
IL, IR‐SEC |
|
|
6a |
|
R=H, Me |
CO/H2O HCO2 − H2 |
19 25 24 |
ETM (CO) ETH (HCO2 −) |
prop. |
n.a. |
|
|
6b |
|
X= –, Cl |
CO/H2O HCO2 − H2 |
73 40 27 |
ETM (CO) ETH (HCO2 −) |
prop. |
n.a. |
|
|
6c |
|
R=C6H4OMe |
CO/CO3 2− |
53 |
ETM |
comp. |
DFT |
|
|
6d |
|
R=C6H4OMe |
CO/CO3 2− |
25 |
ETM |
comp. |
DFT |
|
|
7 |
[Os(CO)(di‐R‐bpy)(L)Cl2] |
R=H, CH3, tBu, CO2‐iPr L=CO, PrCN, Cl |
CO/CO3 2− HCO2 − |
60 [219] 48 [219] |
n.a. |
n.a. |
n.a. |
|
|
|
|
|
|
|
|
|
|
|
|
8 |
[Os(CO)(bpy)2H]+ |
– |
CO/H2O HCO2 − |
90 [221] 25 [221] |
ETM |
exp. |
IL |
[a] After addition of (CH3)4NBF4. [b] After addition of HNMe2. [c] 2,9‐Dimethyl‐1,10‐phenanthroline. [d] 1‐Methylbenzimidazol‐2‐ylidene‐3‐(2′‐pyridine).