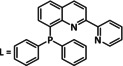
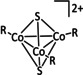
Table 6.
Catalytic systems, major products, maximum FEs, and mechanisms of Co complexes in electrochemical CO2 reduction (n.a.=not available, prop.=proposal, comp.=computational investigation, exp.=experimental evidence).
|
Entry |
Cat. system |
Substitution |
Major product |
Max. FE (%) |
Mechanism |
Basis |
Method |
Ref. |
|---|---|---|---|---|---|---|---|---|
|
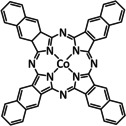
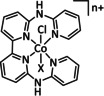
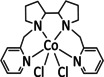
1a |
|
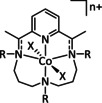
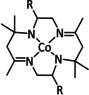
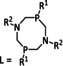
n=1, 2 R= –, H, Me X= –, MeCN, NCS, Cl, Br |
CO/H2O H2 |
45±7 [235] 30±8 [235] |
ETM |
exp.[236 comp. [237] |
IL, IR DFT |
|
|
1b |
|
– |
CO/H2O |
82 |
ETM |
comp. |
DFT |
|
|
1c |
|
– |
HCO2H |
80 |
ETM (CO) ETH (HCO2H) |
exp. comp. |
IR‐SEC DFT |
|
|
1d |
|
– |
HCO2H |
60 |
n.a. |
n.a. |
n.a. |
|
|
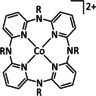
2a |
|
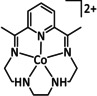
R=H, Me |
CO/H2O H2 |
93 (CO+H2) |
ETH |
prop. |
n.a. |
|
|
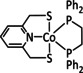
3a |
|
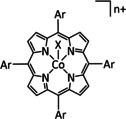
Ar=Ph, C6H4CF3, C6H4NH2, C6H4NMe3 +, C6H3(OH)2, C6H4OMe, C6H3(OMe)2, C6H4Cl, C6H4Br, C6H4F, C6F5 n=0, 1 X= –, Cl |
CO/H2O |
97 [242] |
ETM |
prop. [242] |
n.a. |
|
|
3b |
|
– |
CO/H2O |
89 |
ETM |
prop. |
n.a. |
|
|
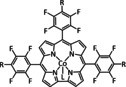
3c |
|
R1=H, tBu, OC8H17, O‐py R2=H, tBu, OC8H17, O‐py R3=H, NMe3 + |
CO/H2O H2 MeOH |
100 [120] 72 [120] 20 [231] |
ETM |
exp. [230] comp. [245] |
PSCAS DFT |
[120, 230, 231, 243e, 245, 246] |
|
3d |
|
– |
CO |
97 |
n.a. |
n.a. |
n.a. |
|
|
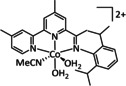
4 |
|
L=PPh3 R=S‐(PEG7)‐OCH3 |
HCO2 − CH3OH H2CO CH3CH2OH CH3CO2 − H2 |
12 59 10 48 13 36 |
ETM |
exp. |
EPR, GC‐MS, IL, IR‐SEC, NMR |
|
|
5a |
|
– |
CO/H2O |
77 |
n.a. |
n.a. |
n.a. |
|
|
5b |
|
R=CH3 R∩R=CH2, (CH2)2 |
CO/H2O |
98 |
ETM |
comp. |
DFT |
|
|
5c |
|
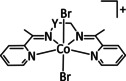
n=0, 1 X= –, Cl |
CO/H2O |
16 |
ETM |
exp. comp. |
IR, UV/Vis DFT |
|
|
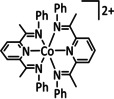
6 |
|
R=H, Me, allyl |
CO/H2O |
98 |
ETM |
comp. |
DFT |
|
|
7 |
|
L=py, qui (+ dimer) |
CO/H2O |
84 |
ETM |
comp. |
DFT |
|
|
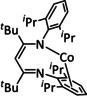
8 |
|
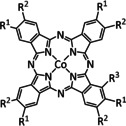
R1=H, tBu, OMe, p‐Me‐C6H4, p‐Cl‐C6H4 R2=H, tBu |
CO/H2O H2 |
37 [253] 23 [253] |
ETM |
prop. [253] |
n.a. |
|
|
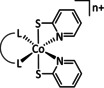
9 |
[Co(PPh3)2L]n+ |
L=4,4′‐di‐Me‐bpy, 4‐Me‐1,10‐phen, 2‐Me‐8‐hydroxyqui n=1, 2 |
CO/CO3 2− HCO2 − |
83 44 |
ETM |
prop. |
n.a. |
|
|
|
|
|
|
|
|
|
|
|
|
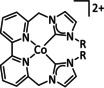
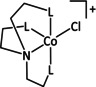
10a |
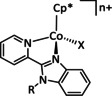
[CoCp(L)I]+ |
R1=Cy, Ph R2=Ph, Bn |
HCO2 − H2 |
99±8 67±5 |
ETH |
comp. |
DFT |
|
|
|
|
|
|
|
|
|
|
|
|
10b |
|
n=1, 2 R=H, Me X=MeCN, I |
CO/H2O |
70 |
ETM |
prop. |
n.a. |
|
|
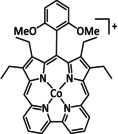
11a |
|
– |
CO/H2O |
94 [184] |
ETM |
prop. [184] |
n.a. |
[184, 229, 258] |
|
11b |
|
Y=CH2, (CH2)2, (CH2)3 |
CO/H2O HCO2H |
104±6 [259] 23 [260] |
ETM |
prop. |
n.a. |
|
|
12 |
|
– |
CO/H2O |
96 |
ETM |
comp. |
DFT |
|
|
13a |
|
– |
CO/H2O |
95±2 |
ETM |
comp. |
DFT |
|
|
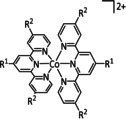
13b |
|
L∩L=dppe, bpy, 4,4'‐(OMe)2bpy, 2‐pyridinethiolato n=0, 1 |
CO/H2O HCO2H H2 |
92±4 [263] (CO+H2) 64 [264] 19 [264] |
ETM (CO) ETH (HCO2H/H2) |
comp. |
DFT |
|
|
14 |
|
– |
n.a. |
n.a. |
ETM |
exp. comp. |
XRD coupled cluster |
|
|
15 |
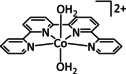
[Co(L)2]2+ |
|
CO/H2O H2 |
23 42 |
ETM (CO) ETH (H2) |
prop. |
n.a. |
|
|
16 |
|
R=MeCp |
C2O4 2− |
80 |
n.a. |
n.a. |
n.a. |